
Welcome to the 16th Issue of Magic Powder!
This edition brings together a focused selection of scientific studies, community highlights, and creative content from across our catalysis network. From NCC-10, we feature research on biochar–MOF glucose sensors, machine-learning approaches to CO₂ electrolysis, low-temperature OCM with nanowire catalysts, pulse-fed ammonia synthesis, methanol reaction mechanisms, and a practical method for synthesizing size-controlled copper nanoparticles.
Our impressions from UKMK-16 reflect the strong momentum of the national chemical engineering community, where sustainability, digitalization, and interdisciplinary research were at the forefront of discussions.
We also highlight recent publications from our researchers spanning Fischer–Tropsch synthesis, computational catalysis, sustainable aerogels, hydrogen and syngas production, polymer upcycling, and semiconductor interface engineering.
This issue includes a new contribution to our PhD Stories series, offering an insightful look into the intellectual and experimental journey behind doctoral research. Following this, we continue with selections from Professor Merlin Catalystorius’ Catalytic Wisdom Guide and our catalysis-themed crossword puzzle, adding a touch of creativity and entertainment.
Finally, our announcements section shares exciting news about the upcoming ACS-8 & RKCM + YOURHETCAT 2026 joint conferences, which promises to bring together researchers from all career stages in kinetics, mechanisms, and catalysis.
We thank all contributors and readers for their continued support and wish you an enjoyable reading experience.
The Catalysis Society
Editorial Board
Prof. Dr. Ayşe Nilgün AKIN
Prof. Dr. N. Alper TAPAN
Dr. Merve DOĞAN ÖZCAN
Asst. Prof. Dr. Elif CAN ÖZCAN
Dr. Mustafa Yasin ASLAN
Inside
- Articles selected from NCC-10
- Impressions from UKMK-16: The Gathering of the Chemical Engineering Community in Bolu
- PhD Stories
- By Necdet Semih Altınsoy
- Recent Selected Studies in Our Catalysis Community
- Crossword Puzzle and Selections from the Catalytic Wisdom Guide by Professor Merlin Catalystorius
Articles selected from NCC-10
Functional Carbon-MOF Hybrids: Glucose Sensing Performance of Activated Biochar-ZIF-8 Composites
Şeyma Çalışkan 1, Yousef Alyosefalbakour 1, Yasin Korkmaz 1 & Ayten Ateş 1,*
1 Kimya Mühendisliği Sivas Cumhuriyet Üniversitesi
ates@cumhuriyet.edu.tr
Biochar is a carbon-rich material produced from biomass waste through thermochemical processes such as pyrolysis, hydrothermal carbonization, and gasification [1]. Due to its large surface area and high porosity, biochar has attracted significant attention as an alternative material in electrochemical energy storage and sensing applications [2]. These properties can be tailored by adjusting the activation methods, which critically influence performance. Among various agricultural wastes, hazelnut shells stand out as a locally abundant and underutilized biomass source in regions with high hazelnut production, such as Türkiye. Instead of being incinerated or discarded, utilizing these shells for functional material synthesis offers both environmental and economic advantages.
Zeolitic imidazolate frameworks (ZIFs), such as ZIF-8, are porous crystalline structures composed of metal ions and organic linkers, known for their potential in sensors due to their high surface area and tunable pore structure [3-4]. However, ZIF-8 suffers from limited conductivity, restricting its standalone use in electrochemical applications. This limitation can be addressed by incorporating conductive carbon materials like biochar to form functional composites.
In this study, biochar was synthesized from hazelnut shells (HZN) via pyrolysis after activation using various methods (acidic, basic, and ultrasonically assisted) at 500°C. The resulting biochars were then composited with ZIF-8 as reported in [5], and the electrochemical behavior of these composites was systematically investigated. Electrochemical analyses were conducted using a Gamry potentiostat/galvanostat in a three-electrode setup, where the working electrodes were prepared by drop-casting a suspension of the composites with Nafion binder onto a glassy carbon electrode.
FTIR results indicated an increase in surface hydroxyl groups, particularly in acid-activated samples, while XRF analysis showed Zn content ranging from 0.76% to 37.27%, increasing with ZIF-8 loading. XRD data confirmed the preservation of ZIF-8's crystal structure within the composites, albeit with slight crystallinity reduction due to biochar incorporation. Among all samples, the ZIF-8/HZN-P500 (50:200) composite exhibited the highest current response, which increased proportionally with glucose concentration, indicating promising glucose sensing capabilities. The enhanced performance is attributed to the well-dispersed ZIF-8 on the biochar surface and the favorable microporous structure supporting electrochemical activity.
These findings demonstrate that biochar/ZIF-8 composites, particularly the ZIF-8/HZN-P500 formulation, are promising candidates for glucose sensor applications.
Keywords: Glucose sensor, Zeolitic imidazolate framework (ZIF-8), Biochar, Electrochemical sensor, Biomass waste
Acknowledgment: Yousef Alyosefalbakour and Yasin Korkmaz gratefully acknowledge the support of the TÜBİTAK 2209-A Project for funding
References:
[1] F. Mahmood et al., “A review of biochar production and its employment in synthesizing carbon-based materials for supercapacitors,” Ind Crops Prod, vol. 227, p. 120830, May 2025, doi: 10.1016/J.INDCROP.2025.120830.
[2] S. Qu et al., “Carbon defects in biochar facilitated nitrogen doping: The significant role of pyridinic nitrogen in peroxymonosulfate activation and ciprofloxacin degradation,” Chemical Engineering Journal, vol. 441, p. 135864, Aug. 2022, doi: 10.1016/J.CEJ.2022.135864.
[3] C. Chen, Y. Zhong, S.Y. Cheng, Y.Y. Huanga, T.X. Li, T.L. Shi, G.L. Liao, Z.R. Tang, In-situ fabrication of porous nanostructures derived from bimetal-organic frameworks for highly sensitive non-enzymatic glucose sensors, J. Electrochem. Soc. 167 (2020), 027531, https://doi.org/10.1149/1945-7111/ab6b05.
[4] A. Hosseini Sharifabad, S. A. Safavi-Mirmahalleh, M. Golshan, M. Sienkiewicz, M. R. Saeb, and M. Salami-Kalajahi, “Metal-organic frameworks (MOFs)-based hybrid structures in developing glucose sensors,” Chemical Engineering Journal, vol. 512, p. 161513, May 2025, doi: 10.1016/J.CEJ.2025.161513.
[5] X.R. Chen, D. Lau, G.J. Cao, Y. Tang, C. Wu, In-situ synthesis of sandwich-like Graphene@ZIF-67 heterostructure for highly sensitive nonenzymatic glucose sensing in human serums, ACS Appl. Mater. Interfaces 11 (2019) 9374–9384, https://doi.org/10.1021/acsami.8b22478.
Exploration of Operating, Catalytic and Material Conditions towards CO in CO2 Electrolyzers: A Machine Learning Study
Niyazi Alper Tapan 1,* & M. Erdem Günay 2
1Kimya Mühendisliği Gazi Üniversitesi
2Enerji Sistemleri Mühendisliği Istanbul Bilgi Üniversitesi
atapan@gazi.edu.tr
The capture, conversion, and valorization of CO₂ play a pivotal role in combating global warming and mitigating the impacts of climate change. Varying CO2 feed stocks from heavy industries to atmospheric air can be converted by electrolyzers to CO which is a bridge towards higher hydrocarbons, alcohols and liquid fuels. Although there is an advantage of transforming CO2 to syngas through electrolyzers with low energy requirements against thermochemical pathways [1] (dry reforming, reverse water gas shift, methanation and thermochemical splitting), commercial readiness still needs optimization of electrolyzer from the point of cell design, electrodes, membrane, electrocatalyst, electrolyte and operation conditions etc. and still needs exploration of complex interactions between these operating, material and catalytic conditions which can be handled by machine learning (ML) algorithms. Although CO as a key electrolyzer reduction product towards selectivity of multicarbon products by C-C coupling and is well known as critical surface intermediate by DFT studies on transition metals [2,3], the selectivity of CO in an electrolyzer involves many factors as emphasized by our previous classification ML models based on a global CO2 reduction dataset. Our previous ML model discovered different routes with nodes of different transition metals, applied potential, cell designs, operating conditions, membrane type and electrolyte composition [4].
In this study, it was decided to develop our previous study further to search for hidden optimum conditions for maximum selectivity, search for the effect and importance of key factors on selectivity with new data mining tools (visualization tools, feature engineering, ML optimization algorithms) [5,6,7,8]. Visualizations and principal component analysis gave suggestions to experimenters about selection of certain cathode elements (Pd,Ag, Cu, Au), GDL types (Sigracet, TGP), membrane type (N212), certain anolyte concentrations (KCl) for high selectivity and warn about conditions toward low selectivity. Boruta analysis through highest accurate ML regressor indicate cathode elemental compositions, pH, potential, CO2 flux dominate faradaic efficiency. GBR-PSO optimization algorithm extracted hidden maximum in the CO2 reduction dataset at high pH (13), high potential (2,97V) and high CO2 flux (48ml/min.cm2) with multielemental cathode electrode. Partial dependence plots from DNN architecture of 9 hidden layers after hyperoptimization with a 8.8% RMSE testing accuracy indicate optimal conditions for Cu at.%, pH and CO2 flux, positive and negative correlations with other operating, material and catalytic conditions and indicate that cathode surface at. compositions, CO2 flux and applied potential dominate on the CO selectivity.
Keywords: electrolyzer, carbon dioxide, carbon monoxide, machine learning, data mining
References:
[1] O’Brien, C. P., Miao, R. K., Shayesteh Zeraati, A., Lee, G., Sargent, E. H., & Sinton, D. (2024). CO2 electrolyzers. Chemical Reviews, 124(7), 3648-3693.
[2] Feaster, J. T., Shi, C., Cave, E. R., Hatsukade, T., Abram, D. N., Kuhl, K. P., ... & Jaramillo, T. F. (2017). Understanding selectivity for the electrochemical reduction of carbon dioxide to formic acid and carbon monoxide on metal electrodes. Acs Catalysis, 7(7), 4822-4827.
[3] Kuhl, K. P., Hatsukade, T., Cave, E. R., Abram, D. N., Kibsgaard, J., & Jaramillo, T. F. (2014). Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. Journal of the American Chemical Society, 136(40), 14107-14113.
[4] Günay, M. E., Türker, L., & Tapan, N. A. (2018). Decision tree analysis for efficient CO2 utilization in electrochemical systems. Journal of CO2 Utilization, 28, 83-95.
[5] Jolliffe, I. T., & Cadima, J. (2016). Principal component analysis: a review and recent developments. Philosophical transactions of the royal society A: Mathematical, Physical and Engineering Sciences, 374(2065), 20150202.
[6] Sabery, G. A., Danishyar, G. H., & Mubarez, G. S. (2023). A comparative study of metaheuristic optimization algorithms for solving engineering design problems. Journal of Mathematics and Statistics Studies, 4(4), 56-69.
[7]https://www.mathworks.com/help/deeplearning/ug/train-network-on-data-set-of-numeric-features.html
[8]https://towardsdatascience.com/feature-selection-with-boruta-in-python-676e3877e596.
Oxidative Coupling of Methane (OCM) at Low Temperature Using Nanowire-Structured La₂O₃ and La₂O₂CO₃ Catalysts: High Activity, Selectivity and Stability
Emel Engintepe 1,* & Ayşe Nilgün Akın 1
1Kimya Mühendisliği Kocaeli Üniversitesi
emel.engintepe@kocaeli.edu.tr
The oxidative coupling of methane (OCM) is a promising route for the direct conversion of methane into high-value C₂ hydrocarbons such as ethylene. Compared to conventional multistep processes, OCM has the potential to operate in a single step with lower energy input and environmental impact. However, the industrial application of OCM remains limited due to high operating temperature requirements (>750 °C) and poor C₂ selectivity [1-3]. Therefore, the development of new catalysts that enable low-temperature activation of methane with high selectivity is of significant interest [4].
In this study, nanowire-structured La₂O₃ and La₂O₂CO₃ catalysts were synthesized via hydrothermal methods and systematically investigated for their performance in the low-temperature OCM reaction. The catalysts were prepared using aqueous ammonia as the precipitating agent under varying hydrothermal conditions (120–220 °C for 12–24 h), followed by calcination at 500 °C and 700 °C. The structural and morphological properties were characterized using X-ray diffraction (XRD), Brunauer–Emmett–Teller (BET) surface area analysis, CO₂ temperature-programmed desorption (CO₂-TPD), and field-emission scanning electron microscopy (FE-SEM).
Catalytic activity was evaluated in a fixed-bed microreactor system coupled with gas chromatography (GC). As shown in Figure 1, the catalyst prepared at 200 °C for 16 h and calcined at 500 °C exhibited the highest performance, achieving 13,1 % C₂+ selectivity at 500 °C. In addition, the long-term performance and structural stability of nanowire-structured La₂O₂CO₃ catalysts were systematically investigated in a low-temperature OCM environment. The reaction conditions were CH₄/O₂/N₂ = 72/24/4, with a total flow of 100 mL/min. The stability test was carried out at 500 °C over 50 hours of continuous operation and given in Figure 2. The La₂O₂CO₃ nanowire catalyst demonstrated remarkable stability during the test, maintaining CH₄ conversion above 30% and C₂+ selectivity around 42% with minimal fluctuations. These findings suggest that La₂O₂CO₃ nanowires exhibit sufficient long-term stability and selectivity, making them strong candidates for low-temperature OCM applications.
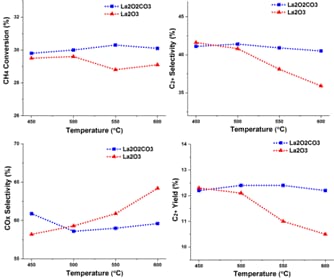
Figure 1. Catalytic activities of La2O2CO3 and La2O3 nanowire catalysts.
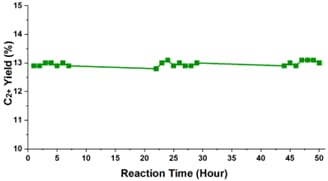
Figure 2. Stability test of La2O2CO3 nanowire catalyst.
This work is among the first to provide comprehensive insight into the long-term performance of La₂O₂CO₃-based nanowire catalysts in low-temperature OCM
Keywords: Nanowire catalyst, long-term stability, low temperature OCM, hydrothermal synthesis
References:
[1] Cruchade, H., Medeiros-Costa, I. C., Nesterenko, N., Gilson, J. P., Pinard, L., Beuque, A., & Mintova, S. (2022). Catalytic routes for direct methane conversion to hydrocarbons and hydrogen: current state and opportunities. ACS Catalysis, 12(23), 14533-14558.
[2] Wang, P., Zhao, G., Liu, Y., & Lu, Y. (2017). TiO2-doped Mn2O3-Na2WO4/SiO2 catalyst for oxidative coupling of methane: Solution combustion synthesis and MnTiO3-dependent low-temperature activity improvement. Applied Catalysis A: General, 544, 77-83.
[3] Karakaya, C., Zhu, H., Zohour, B., Senkan, S., Kee, R. J. (2017). Detailed reaction mechanisms for the oxidative coupling of methane over La2O3/CeO2 nanofiber fabric catalysts. ChemCatChem, 9(24), 4538-4551.
[4] Pal, R. S., Rana, S., Sharma, S. K., Khatun, R., Khurana, D., Khan, T. S., Poddar M.K., Sharma R., Bal, R. (2023). Enhancement of oxygen vacancy sites of La2-xMxCe2O7-δ (M= Ca, Ba, Sr) catalyst for the low temperature oxidative coupling of Methane: A combined DFT and experimental study. Chemical Engineering Journal, 458, 141379.
Ammonia Synthesis through a Pulse Feeding Strategy Reveals Ammonia Desorption as the Major Bottleneck in the Reaction
Mustafa Yasın Aslan 1 & Deniz Üner 2,*
1 Kimya Mühendisliği Uşak Üniversitesi
2 Kimya Mühendisliği Orta Doğu Teknik Üniversitesi
uner@metu.edu.tr
Sustainable ammonia production represents a significant challenge for the 21st century, primarily because the Haber-Bosch process consumes substantial amounts of energy and resources, contributing to global pollution [1,2]. One of the proposed solutions to this issue is the implementation of an unsteady-state feeding strategy for the reactants to overcome H2 poisoning effect in the presence of Ru-based ammonia synthesis catalysts [3-5]. This approach can enhance the instantaneous ammonia synthesis rate on the catalyst surface, thereby improving the overall reaction efficiency. From a thermodynamic point of view, although offers the potential to achieve higher product yields per pass and to eliminate several cost factors, such as those associated with gas compression, the probability of condensation of ammonia on the pores/surface of the catalyst and inside/outlet of the reactor should not be ignored due to the thermophysical properties of ammonia.
In this study, ammonia synthesis reaction over 1 wt% Ru/SBA-15 catalyst was carried out by applying steady and unsteady state feeding strategies. 1 wt % Ru/SBA-15 catalyst was prepared via incipient wetness method. The characterization studies of the Ru/SBA-15 catalyst showed the presence of Ru nanoparticles both on the surface and within the pores of the SBA-15 support. The steady-state experiments demonstrated consistent ammonia production and stable NH₃ signal response. In contrast, transient-state experiments indicated that ammonia desorption from the catalyst surface and reactor can represent a significant bottleneck for ammonia synthesis under mild operating conditions due to the thermophysical properties of ammonia.
Keywords: Ammonia Synthesis, Ru, Unsteady State Feeding
References:
[1] I. Rafiqul, C. Weber, B. Lehmann and A. Voss, "Energy efficiency improvements in ammonia production-perpectives abd uncertainties," Energy, 30 (13), 2005, pp. 2487-2504
[2] N. Saadatjou, A. Jafari and S. Sahebdelfar, "Ruthenium nanocatalysts for ammonia synthesis: A review," Chemical Engineering Communications, 202, (4), 2015, pp. 420-448
[3] G. Rambeau and H. Amariglio, “Improvement of the catalytic performance of a ruthenium powder in ammonia synthesis by the use of a cyclic procedure,” Applied Catalysis, vol. 1, no. 5, pp. 291–302, Apr. 1981.
[4] G. Rambeau, A. Jorti, and H. Amariglio, “Improvement of the catalytic performance of an osmium powder in ammonia synthesis by the use of a cyclic procedure,” Applied Catalysis, vol. 3, no. 3, pp. 273–282, Nov. 1982.
[5] H. Amariglio and G. Rambeau, “On the possibility of rate improvements by a periodic operation of catalytic reactors,” Chemical Engineering Science, vol. 39, no. 9. pp. 1433, 1984.
Mechanistic Insights into Methanol Formation and Decomposition via First-Principles Simulations
Murat Oluş Özbek
Kimya Mühendisliği Gebze Teknik Üniversitesi
olus.ozbek@gtu.edu.tr
Methanol (CH₃OH) is an important molecule in the clean energy landscape, offering versatile utility as a liquid fuel, hydrogen carrier, and chemical feedstock [1]. Its formation through syngas (CO/CO₂ + H₂) [2] conversion supports renewable fuel production, while its decomposition provides a practical route to on-demand hydrogen generation—particularly important for high-temperature proton exchange membrane (PEM) fuel cells [3]. Advancing these applications requires a deep mechanistic understanding of surface reactivity and selectivity at the atomic scale.
Through a series of studies, we employ density functional theory (DFT) to investigate the formation and decomposition of methanol on copper and zinc surfaces. For methanol synthesis, we explored CO and CO₂ hydrogenation pathways on Cu(100), (110), (111), and stepped (211) surfaces, as well as Zn(0001), (1000), and (1100) surfaces. All plausible reaction routes—including C–O bond cleavage and intermediate hydrogenation steps—were analyzed. Our goal is to understand the selectivity shift in industrial Cu/ZnO catalysts from CO to CO₂ hydrogenation [4,5], given the known difficulty of C–O bond cleavage on bare Cu. In addition, single-atom doping effects (Cu in Zn, and vice versa) were investigated to assess local compositional impacts. These insights offer atomistic explanations for experimental trends observed in Cu/ZnO catalyst systems and represent one of the most comprehensive DFT-based evaluations of methanol-related mechanisms to date.
We also examined methanol decomposition on clean and Zr-modified Cu(100) and Cu(111) surfaces, motivated by experimental evidence showing enhanced catalytic performance with Zr and ZrO₂ additions [6]. Specifically, we modeled copper surfaces modified with single Zr atoms and small ZrO₂ clusters and compared them to the clean surfaces. Our simulations provide theoretical validation of the experimentally observed enhancement in methanol decomposition selectivity and reveal how metal–oxide interfaces affect dehydrogenation energetics, promote selective H₂ evolution while suppressing CO formation—crucial for fuel cell integration.
Altogether, this work bridges theoretical surface science with applied catalyst design, offering atomic-level insights to guide the development of selective and efficient methanol-based energy technologies.
Keywords: Methanol, DFT, catalysis, mechanism
References:
1: Ullah, A.; Hashim, N.A.; Rabuni, M.F.; Junaidi, M.U.M. Methanol as a Clean Energy Carrier: Roles of Zeolite in Improving Production Efficiency. Energies 2023, 16(3), 1482.
2: Jia, Y.; Zhao, Y.; Chen, Y.; Zhao, W.; Zhang, X.; Yang, Z. Advances in Catalytic CO₂ Hydrogenation to Methanol: From Catalysts to Processes. Catalysts 2022, 12(4), 403.
3: Liu, H.; Du, J.; Shen, Y.; He, Y. A Review on Methanol Reforming for Hydrogen Production: Current Status and Future Perspectives. Chemical Engineering & Technology 2023, 46(4), 537–553.
Araya, S. S.; Liso, V.; Cui, X.; Li, N.; Zhu, J. A Review of the Methanol Economy: The Fuel Cell Route. Energies 2020, 13(3), 596.
4: Fujitani, T.; Nakamura, J.; Uchijima, T.; Nakamura, J. The Role of ZnO in Methanol Synthesis over Cu/ZnO Catalysts. Catalysis Letters 1998, 56(1), 119–124.
5: Xu, D.; Wu, P.; Yang, B. Origin of CO₂ as the Main Carbon Source in Syngas-to-Methanol Process over Cu: Theoretical Evidence from a Combined DFT and Microkinetic Modeling Study. Catalysis Science & Technology 2020, 10(15), 5176–5186.
6: Jeong H, Kim KI, Kim TH, Ko CH, Park HC, Song IK. Hydrogen production by steam reforming of methanol in a micro-channel reactor coated with Cu/ZnO/ZrO2/Al2O3 catalyst. Journal of Power Sources 2006;159:1296–9.
Controlled Size Distribution of Copper Nanoparticles via a One-Pot Synthesis Approach
Rahime Aybike Koraş 1, Fatma Eda Özgüven 2 & Mustafa Karatok 2,*
1 Nanobilim ve Nanoteknoloji Hacettepe Üniversitesi
2 Nanoteknoloji ve Nanotıp Hacettepe Üniversitesi
mustafakaratok@hacettepe.edu.tr
Copper has attracted considerable attention in recent years as a catalyst due to its low cost, earth abundance and low toxicity. Cu nanoparticles are utilized in a broad spectrum of organic transformations, including azide–alkyne cycloaddition (“click chemistry”), redox reactions, cross-coupling, C–H functionalization, borylation, and oxidative coupling. [1] Beyond organic synthesis, Cu-based catalysts also find applications in environmentally and energetically critical processes, such as NOₓ reduction, CO oxidation, and the water–gas shift reaction.[1] However, despite its versatility and promise, copper’s catalytic activity remains limited in many reactions due to its relatively low inherent reactivity toward organic substrates. To overcome this limitation, small amounts of more reactive metals such as Pt or Pd are often alloyed with copper. This strategy has demonstrated significant improvements in catalytic performance across a variety of reactions.
Copper nanoparticles often function as the host component in bimetallic systems to enhance the product selectivity, while the more reactive metal initiates the reaction cycle through bond cleavage. It plays a crucial role in modulating reaction pathways by weakening the adsorption strength of intermediate species, thereby suppressing undesired side reactions and improving selectivity toward target products. [2] For instance, PtCu nanoparticles have been shown to increase the selectivity toward formate by 74% in the electrochemical CO2 reduction reaction. [4]
Bimetallic alloys are inherently dynamic systems, and their surface composition can vary significantly depending on the reaction environment. Under certain conditions, active metal atoms may irreversibly diffuse into the bulk of the host metal, leading to a loss of surface- active sites and consequent catalyst deactivation. This surface-to-bulk redistribution is strongly influenced by both the alloy composition and the particle size of the host material. In particular, the stability and catalytic performance of Cu-based alloys are highly sensitive to the size of the copper nanoparticles. However, achieving controlled synthesis of copper nanoparticles remains a significant challenge due to copper’s high susceptibility to oxidation and the pronounced sensitivity of its nucleation and growth processes to subtle changes in reaction parameters. Precise control over particle size and alloy composition often requires intricate synthetic strategies involving careful adjustment of multiple variables, making the preparation of well-defined Cu-based nanostructures a technically demanding task. [1], [2], [5]
In this study, we developed a straightforward, one-pot synthesis method for producing metallic Cu nanoparticles with controlled particle sizes. By systematically varying key synthetic parameters, including the choice of reducing agent, capping agent, precursor concentration, and injection rate, we achieved size tunability across a broad range, from 20 nm to 1 µm, as confirmed by SEM analysis. The use of strong reducing agents such as NaBH4, faster reduction rates, or higher concentration of capping agents (e.g., PVP) led to the formation of smaller particles. In contrast, employing weaker reducing agents like ascorbic acid or slowing the reduction process, even with a strong reducing agent, resulted in the formation of larger particles. This simple approach provides a robust platform for systematically investigating the role of copper particle size in Cu-based alloy systems. The ability to synthesize various sizes of pure Cu nanoparticles using a consistent protocol allows for controlled doping of active metals, facilitating detailed studies on how Cu particle size influences catalytic performance across different reactions.
Keywords: Copper Catalysts, Nanoparticle Synthesis, One-Pot Synthesis
References:
[1] Gawande, M. B., Goswami, A., Felpin, F. X., Asefa, T., Huang, X., Silva, R., ... & Varma, R. S. (2016). Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chemical reviews, 116(6), 3722-3811.
[2] Lee, J. D., Miller, J. B., Shneidman, A. V., Sun, L., Weaver, J. F., Aizenberg, J., ... & Friend, C. M. (2022). Dilute alloys based on Au, Ag, or Cu for efficient catalysis: from synthesis to active sites. Chemical reviews, 122(9), 8758-8808.
[3] El Berch, J. N., Salem, M., & Mpourmpakis, G. (2025). Advances in simulating dilute alloy nanoparticles for catalysis. Nanoscale, 17(4), 1936-1953.
[4] Gutiérrez-Roa, M., Sebastián, D., Guzmán, H., Zammillo, F., Gallone, M., Hernández, S., ... & Pérez-Rodríguez, S. (2025). Tuning the activity and selectivity of CuPt/C catalysts for the electrochemical CO2 reduction. Journal of CO2 Utilization, 95, 103084.
[5] Acquaye, F. Y., Roberts, A., & Street, S. (2022). Effect of crystal growth on the thermodynamic stability and oxygen reduction reaction activity of Cu–Pt nanoparticles. Langmuir, 38(34), 10621-10631.
Impressions from UKMK-16: The Gathering of the Chemical Engineering Community in Bolu
The 16th National Chemical Engineering Congress (UKMK-16), hosted by Bolu Abant Izzet Baysal University between September 9 and 12, 2025, brought together the chemical engineering community of our country, providing a productive platform for scientific exchange, education, and industry collaboration.
A total of 246 participants shared 99 oral and 66 poster presentations across 36 sessions over four days. The congress provided an opportunity to discuss current trends in chemical engineering with participants from various disciplines.
In the opening sessions, Dr. Belma Soydaş Sözer, President of TENMAK Rare Earth Elements Research Institute (NATEN), emphasized the strategic importance of sustainable mining in her presentation titled "Global Competition in Critical Raw Materials: Turkey's R&D and Production Vision in the Rare Earth Elements Ecosystem." Following her, Dr. Berna Yüksel, President of TENMAK Boron Research Institute (BOREN), evaluated new trends in boron technologies in her speech titled "Global Scenarios in Boron Research and Applications and Common Sense in Turkey's Boron Strategy."
One of the keynote speeches that marked the international dimension of the congress was delivered by Prof. Ahmed Ghoniem from the Department of Mechanical Engineering at the Massachusetts Institute of Technology (MIT). Ghoniem addressed hydrogen production technologies based on redox cycles, aiming to convert solar energy directly into chemical energy, in his presentation titled "Solar Thermochemical Hydrogen Production Using Redox Active Material." Prof. Rafiqul Gani from the Technical University of Denmark (DTU) drew attention to the transformative effects of digitalization on process design and optimization in his talk titled "Chemical Engineering in the Age of Computers and Artificial Intelligence."
Within the scope of the congress, a special session titled "National Chemical Engineering Congress in its 30th Year," held in honor of Prof. Dr. Canan Özgen, discussed the development of the field through historical and contemporary perspectives. Presentations in this session, including "The Past, Present, and Future of Chemical Engineering," "The Impact of Artificial Intelligence on Education, Scientific Research, and Industry," and "Development of Interdisciplinary Research in Chemical Engineering," laid the groundwork for important discussions on both the renewal of education and the expansion of research areas. In the Chemical Reaction Engineering Education session chaired by Prof. Dr. Ahmet Kerim Avcı, a wide range of topics related to catalysts and chemical reactions — spanning from past developments to future perspectives — were comprehensively discussed.
In addition, special sessions on Fluid Mechanics Education, Chemical Reaction Engineering, and the Chamber of Chemical Engineers (KMO) addressed how fundamental engineering courses are being harmonized with new technologies and the role of industry collaboration in education.
Key themes highlighted in the congress declaration included sustainability, green transformation, digitalization, circular economy, and interdisciplinary collaboration. Participants agreed that chemical engineering is increasingly taking a central position in areas such as energy transition, environmental technologies, and advanced materials development.
UKMK-16 facilitated the establishment of lasting connections among young researchers, academics, and industry representatives alongside scientific exchange. This event, which supports scientific productivity and a culture of collaboration, created a solid foundation for future collaborations and idea exchange in the field of chemical engineering.
PhD Stories
By Necdet Semih Altınsoy
My first research topic was to model direct synthesis of DME from CO and H2 inside a microchannel reactor, which includes methanol synthesis reaction from CO and H2, methanol dehydration reaction and water-gas shift reaction. Our team SPICE also had required equipment to conduct this synthesis experimentally. In this way, I had the opportunity to conduct a reaction, which can be enhanced by taking place inside a pressurized reactor. In fact, this means a quite good opportunity for application of what we had learnt during reaction kinetics and reactor design lecture. Decrease in the reactor exit flow rate compared to inlet flow rate can be observed physically depending on the conversion inside the reactor. Carbon source of this system was changed from CO to CO2, to utilize CO2, which is a greenhouse gas. When carbon source of the system was CO, H2O formed as a result of methanol dehydration reaction could be converted to the H2 via water-gas shift reaction to remove H2O from the reactor and to drive methanol dehydration reaction forward. When CO2 is carbon source, this could not be done. Moreover, reverse water-gas shift reaction also produces water beside methanol dehydration reaction. Since water could not be removed inside the reactor via water-gas shift reaction, yield of the system could not be increased. In this case, to increase yield of the system by removing H2O, a H2O selective adsorbent should be used but adsorbents have certain adsorption capacity and this adsorption capacity is occupied after a while from the start of the reaction. As an advantage of a pressurized system, adsorbent can be regenerated by decreasing pressure of the system. All in all, producing DME with high yield strongly depends on carbon source and H2O adsorpsion. Indeed, when carbon source is CO2, the more H2O is adsorbed, the more carbon source becomes as if CO and this emphasizes importance of reverse water-gas shift reaction. This experience was the determining factor for my PhD thesis proposal, which is enhancing reverse water-gas shift reaction with adsorbent usage and regeneration of adsorbent. Actually, regeneration of adsorbent is a vast subject. Reverse water-gas shift reaction could not be enhanced with pressure as if in methanol synthesis. In this respect, only benefit of using pressurized reactor can be increased adsorption capacity of adsorbent; however, with pressurized system, dead volume of the system increases and for the productivity calculations, dead of the system should be taken into account. This has a negative effect on productivity of system, which is product mass per time and per catalyst weight. Since we already know, this reaction could not be enhanced by pressure, conducting this reaction at atmospheric pressure and regenerating adsorbent with temperature swing seem like a good idea. Using conventional oven limits productivity of system because of limitations in temperature increase and decrease capabilities of conventional ovens. With electrification, by heating a really small amount of mass, a significant increase in temperature ramp capabilities of system, both as increase and decrease, can be obtained. Using induction, which is one of the electrification methods, reactor can be heated without any physical contact. Other important difference of induction heating from conventional ovens, in which heat transfer is from outside to inside, is that heat transfer is from inside to outside. Heating inside the reactor takes place on the susceptor. Heat released from susceptor when induction is applied depends on the characteristics of susceptor and for efficient heating, susceptor should be a material that releases enough heat. To improve productivity of reverse water-gas shift reaction, our studies utilizing susceptors that we developed are ongoing.
Recent Selected Papers in Our Catalysis Community
Fischer–Tropsch Synthesis
Akin A. N., Özcan O., Doğan Özcan M., Üner D. (2025). Activation of CO, CO2, and H2 Toward Synthetic Fuel Manufacture by Fischer–Tropsch Synthesis. Catalytic Activation of Small Molecules, Royal Society of Chemistry, ss.158-222, 2025.
This book chapter highlights Fischer–Tropsch synthesis and CO/CO₂ hydrogenation as key routes for sustainable fuel and chemical production in a transition away from fossil resources. It discusses traditional Co, Fe, and Ru catalysts and recent advances such as Co nanoparticles, Fe carbides, single-atom catalysts, Fe₃O₄ nano catalysts, and MOFs that enhance selectivity, efficiency, and lower CO₂ emissions. Integrating CO₂ into syngas processes and deploying these advanced catalysts supports carbon recycling, optimized energy use, and a more sustainable global energy system.
Molecular-Level Understanding via Computational Chemistry
Doğan Ulu, Ö., Serin, S., Özdemir, N., Özdemir, İ. (2025). Synthesis, crystal structure, and DFT studies of NHC mediated Pd-PEPPSI complex: Application for Suzuki reaction. Journal of Molecular Structure, 1320 (2025) 139479.
A new air- and moisture-stable Pd-PEPPSI complex was synthesized and fully characterized by spectroscopy and single-crystal X-ray diffraction. DFT, NBO, and TD-DFT studies were used to understand its structural, bonding, electronic, and thermodynamic properties, as well as the nature of its electronic transitions. This complex showed high catalytic activity in Suzuki–Miyaura cross-coupling of various aryl chlorides with arylboronic acids under green, aerobic conditions with very low catalyst loading, providing biaryl products in high yields.
Sustainable Materials Engineering
García-González, C. A., Blanco-Vales, M., Barros, J., Boccia, A. C., Budtova, T., Durães, L., Erkey, C., Gallo, M., Herman, P., Kalmár, J., Iglesias-Mejuto, A., Malfait, W. J., Zhao, S., Manzocco, L., Plazzotta, S., Milovanovic, S., Neagu, M., Nita, L. E., Paraskevopoulou, P., Roig , A., Simón-Vázquez, R., Smirnova, I., Tomović, Ž., López-Iglesias, C. (2025). Review and Perspectives on the Sustainability of Organic Aerogels, ACS Sustainable Chem. Eng., 13, 6469−6492.
Aerogels are ultra-light, highly porous materials with vast industrial potential, particularly for energy-saving, environmental, and resource-efficient applications that align with global sustainability needs. This Perspective critically reviews recent advances in organic and hybrid aerogels, highlighting case studies tied to UN SDGs and emphasizing strategies like using biobased or waste-derived precursors, improving processing efficiency, and enabling reuse, recycling, and proper end-of-life management. It underscores the urgent need for life cycle assessment, safety, and toxicity evaluations, concluding that aerogels hold strong promise for a circular, sustainable economy from both commercial and research standpoints.
Hydrogen & Syngas Production
Aslan, M. Y. (2025). Steam Reforming and Dry Reforming of Methane. Catalytic Activation of Small Molecules, Royal Society of Chemistry, p.1-39.
The chapter reviews steam and dry reforming of methane for sustainable hydrogen production, covering thermodynamics, side reactions, and overall process considerations. It emphasizes recent advances in catalytic materials designed to address key drawbacks, alongside brief discussions of reaction kinetics, surface mechanisms, and DFT studies. It also touches on reactor intensification strategies such as chemical looping, offering a concise outlook on current practice and future directions.
Bak, Y. G., Ergül, S. D., Oǧulgönen, C. G., Uner, D. (2025). Process Intensification of Air Separation and Autothermal Bi-reforming Reactions Enabled by MnO/Mn2O3 Redox Pair. Energy Fuels 2025, 39, 13728−13740.
This work investigates using solar irradiation to drive endothermic chemical looping with manganese (II) oxide, enabling long-term thermal energy storage and utilization. Mn-oxides showed favorable and stable redox behavior for chemical looping air separation and autothermal methane reforming, where oxygen capture generates nitrogen and CH₄ reduction produces hot H₂O/CO₂ streams that supply both heat and feed for syngas production. The integrated, solar-thermal-intensified process is demonstrated as a promising route for renewable hydrogen and syngas generation.
Polymer Upcycling & Waste-to-Chemicals
Ece, H., Aydoğdu, A. C., Al, F., Erkmen, B., Suerkan, A., Ezdesir, A., Alparslan, A., Celik, G. (2025). Catalytic Upcycling of Polyethylene into Naphtha Using Commercial Heterogeneous Catalysts. Chemistry Select, 10, e06207.
This study evaluates commercial hydrotreatment catalysts for hydrogenolysis of polyethylene into naphtha-range hydrocarbons to support plastic circularity. While catalytic operation is required for liquid production, Pt/Al₂O₃ shows low activity (3% liquid), Ni-based and Au–Pd catalysts give higher yields but off-spec naphtha due to high olefin content, and metal-free materials are largely ineffective. The results show current commercial catalysts are inadequate, underscoring the need for purpose-designed catalysts for polymer depolymerization, selective C–C bond cleavage, and improved mechanistic understanding.
Semiconductor & Interface Engineering
Ardalı, E., Jahangiri, H., Solati, N., Yahsi, U., Tav, C., Sennaroglu, A., Kaya, S. (2025). Cation Vacancy-Mediated Ultrafast Hole Transport in CuBi2O4 Photocathodes. ChemSusChem, 18, e202401345.
This study investigates tetragonal CuBi₂O₄ photocathodes by tuning their atomic composition to manipulate p-type character and probe ultrafast valence band hole dynamics. Using combined ex situ/in situ transient absorption spectroscopy and photoelectrochemical measurements, it shows that valence band holes tend to form polarons at cation vacancy sites. These findings provide a detailed picture of ultrafast hole transport and explain how such defect-associated polaron formation hinders photocurrent generation in CuBi₂O₄.
Machine Learning
Tapan, N. A., Günay, M. E. (2025). Multi-objective optimization of PEM electrolyzers using deep neural networks and gradient boost regressor-particle swarm optimization framework. International Journal of Hydrogen Energy,160, 150622.
A PEM electrolyzer database (Ir anode/Pt cathode; 11 descriptor groups, 484 entries) was modeled with deep neural networks to predict current density, power density, power density×polarization, and current density/polarization. Model explainability via permutation feature importance and partial dependence plots showed potential as the dominant driver across all targets, and identified combinations of anode/cathode gas diffusion layers and membranes that optimize performance. Using a GBR–PSO framework, the study extrapolated optimal values beyond the dataset but found no single holistic optimum because ideal cathode support/surface ratios and anode catalyst loadings vary widely by target.

Previous issue answers Newsletter #15:
1. Polysulifde, 2. Operando, 3. Mxene, 4. Hybrid, 5. Facets, 6. SUESC, 7. Kinetics, 8. Elektrospinning, 9. PEMFC, 10. Nanostructure
Announcements
The 8th Anatolian School of Catalysis (ACS-8):

We are pleased to announce that the 8th Anatolian School of Catalysis will be held from April 23rd to 26th, 2026, in Gümüldür, İzmir.
For detailed information about the school, please visit: https://www.katalizdernegi.org/asc8/
We warmly invite all interested participants to join this enriching event.
4th International Conference on Reaction Kinetics, Mechanisms and Catalysisi & 3rd Forum of Young Researchers on Heterogeneous Catalysis:

Dear Colleagues,
In 2026, two major conference series will unite for the first time: the 4th International Conference on Reaction Kinetics, Mechanisms and Catalysis (RKCM 2026), following its successful editions in Budapest (2018, 2024), online (2021), and the 3rd Forum of Young Researchers on Heterogeneous Catalysis (YOURHETCAT 2026), building on the successes of Szeged (2022, 2024). By uniting these two communities, RKCM+YOURHETCAT 2026 will provide a unique opportunity for scientists at all career stages to interact, inspire one another, and foster meaningful collaborations across the fields of kinetics, mechanisms and catalysis.
The conference will take place at the Central European University (CEU) – Nádor Campus in Budapest, in a super-complex venue designed to reflect the interdisciplinary nature of catalysis. The new campus buildings rank among the best in the world, since they were shortlisted for one of the world's most prestigious architecture awards, the RIBA International Prize. Modern lecture halls will host plenary and other scientific talks, interactive spaces will support posters, scientific meetings as well as friend-making mingling sessions for all participants to highlight Hungary’s advanced facilities in energy and catalysis research.
The program will feature plenary lectures, oral & poster presentations and special “pitch” presentations by brave students where they can “sell” their knowledge, products to the jury. We are planning workshops on scientific communication, funding strategies, industry engagement, and great social programs - including a ruin pub tour in the city center - to ensure lively entertainment in the evenings.
Scientists from all career stages as graduate and postgraduate students, postdocs, senior researchers, YEuCAT as well as EFCATS members, and colleagues from both academia and industry are warmly invited.
All participants of RKMC + YOURHETCAT 2026 will be invited to submit their paper to the special section of Reaction Kinetics, Mechanisms and Catalysis journal (Impact factor: 1.7). The papers will be published after standard peer-review. Registration will open soon on the RKMC 2026 website


