
Dear Catalysis Enthusiasts,
We are back with the 13th edition of our Magic Powder newsletter! In this issue, we delve into electrolysis technologies and oxygen evolution reaction (OER) catalysts, which play a key role in a sustainable future. You will learn about the details of the hydrogen economy through the pen of Assoc. Prof. Dr. Alp Yürüm, explore innovative works from the Kocaeli University KARGEL laboratory, and witness the inspiring academic journey of PhD candidate Mert Özden.
We also have some exciting news! The 10th National Catalysis Conference (NCC10) will take place in Sivas from June 25-28, 2025. Don't miss the deadlines to take advantage of early registration and submit your abstracts! The conference will offer presentations by leading experts, opportunities for young researchers, and vibrant discussions on the pulse of the catalysis world. On June 25, 2025, a one-day school (School of Chemical Reaction Engineering) will be held with invited national and international speakers on chemical reaction engineering and the basics of catalysis. For more details: http://ncc10.cumhuriyet.edu.tr
Additionally, in this issue, you'll find a selection of articles summarizing recent works in our community, a puzzle by Professor Merlin Catalystorius, and highlights of international events in 2025. We wish you enjoyable reading and look forward to seeing you at NCC10!
May the magic of catalysis be with you,
The Catalysis Society
Editorial Board
Prof. Dr. Ayşe Nilgün AKIN
Prof. Dr. N. Alper TAPAN
Dr. Merve DOĞAN ÖZCAN Asst. Prof. Dr. Elif CAN ÖZCAN
Dr. Mustafa Yasin ASLAN
Electrolysis Technologies and OER Catalysts for a Green Future
By Alp Yürüm
Materials Science and Nano Engineering Program, Faculty of Engineering and Natural Sciences, Sabancı University
1. Importance of Hydrogen
Hydrogen is increasingly recognized as a cornerstone of a low‑carbon energy future due to its unique combination of properties. As a light, energy‑dense molecule, hydrogen delivers roughly three times the gravimetric energy content of conventional liquid fuels, yet produces no direct CO₂ emissions when used in fuel cells or combustion processes. Its flexibility—as both an energy carrier and storage medium—allows it to bridge gaps across sectors: from long‑duration storage of variable renewable electricity to feedstock for heavy industry and mobility.
Fundamentally, hydrogen functions much like electricity: a secondary energy carrier that must be produced from primary sources, yet offers the advantage of chemical storage. By converting surplus renewable electricity into hydrogen via electrolysis, we can store energy over days, weeks or even seasons—addressing the intermittency of wind and solar power. This “power‑to‑gas” approach not only balances grids but also enables the transport of renewable energy: hydrogen can be produced in regions with abundant renewables and shipped to demand centers where generation is constrained.
Today, hydrogen’s predominant use is as a chemical feedstock. Roughly 90 million tonnes (Mt) were consumed globally in 2020, of which over 70 Mt served pure‑hydrogen applications (e.g., refining and ammonia synthesis), and an additional 20 Mt were blended for processes such as methanol and steel production. These industrial roles highlight hydrogen’s critical function in decarbonizing sectors that are otherwise hard to electrify, such as high‑temperature heat in steelmaking or fertilizer manufacture [1].
Beyond industry, hydrogen’s potential in mobility is gaining traction. According to the International Energy Agency, key applications include (1) reconversion to electricity (albeit with efficiency losses), (2) direct use in industrial processes, (3) injection into existing natural gas networks for blending, and (4) fuel cell vehicles in the transport sector. While battery electric vehicles dominate passenger electrification efforts, fuel cell vehicles (FCVs) offer faster refueling and longer range—attributes especially valuable for heavy‑duty transport, buses, and niche passenger markets in regions such as Japan, South Korea, and parts of Europe.
Moreover, hydrogen’s role in energy security cannot be overstated. By diversifying energy supply pathways—linking electricity, gas, and transport infrastructures—it enhances resilience against price volatility and geopolitical risks. As nations pursue net‑zero targets, hydrogen emerges as a versatile enabler: it stores renewable surpluses, decarbonizes industry, and powers clean mobility, all while leveraging existing energy infrastructure and enabling new value chains.
2. Traditional Hydrogen Production Methods
Historically, nearly all industrial hydrogen has been produced from fossil resources through thermochemical processes. Today, over 96 % of global hydrogen originates from these conventional routes, with steam methane reforming (SMR) of natural gas alone accounting for roughly 83 % of production [1]. Below, the principal methods are summarized (Figure 1):

Figure 1. Various hydrogen production methods [2]
2.1. Steam Methane Reforming (SMR)
SMR remains the workhorse of hydrogen manufacture (Figure 2). In a catalytic reactor, methane (CH₄) reacts with high‑temperature steam (700–1,000 °C) to yield synthesis gas (CO + H₂):
CH4 + H2O ⟶ CO + 3H2 (1)
A downstream water‑gas shift step then converts CO to CO₂ and additional H₂:
CO + H2O ⟶ CO2 + H2 (2)
SMR plants typically operate at large scale (100–1,000 MW) with process efficiencies of 60–85 % (lower heating value basis). The chief drawback is the large CO₂ footprint (≈9–12 kg CO₂ per kg H₂) unless carbon capture is applied.

Figure 2. Schematic production process of Steam reforming of natural gas (SMR)
2.2. Partial Oxidation (POX) and Autothermal Reforming (ATR)
In POX, hydrocarbons (natural gas or heavier fractions) are partially combusted with O₂ to generate heat and syngas:
CH4 + ½O2 ⟶ CO + 2H2
ATR combines POX and SMR in a single reactor, balancing exothermic and endothermic reactions to improve thermal efficiency. Typical efficiencies are 60–75 %, with CO₂ emissions comparable to SMR.
2.3. Coal Gasification
Coal (lignite, sub‑bituminous, bituminous) is gasified with steam and oxygen at >900 °C to produce syngas (CO + H₂), followed by shift and purification. Although coal gasification can leverage abundant feedstocks, efficiencies are similar to SMR (74–85 %) and CO₂ emissions are even higher unless mitigated by CCS.
2.4. Methane Pyrolysis
Thermal cracking of methane in the absence of oxidants yields H₂ and solid carbon:
CH4 ⟶ 2H2 + C(s)
Pyrolysis offers the prospect of low‑carbon hydrogen if the solid carbon can be sequestered or valorized. Technology maturity ranges from pilot to demonstration scale, with efficiencies of 35–50 % reported.
2.5. Biomass‑ and Waste‑Based Routes
Biomass gasification and fermentation (dark or photofermentation) can produce hydrogen with potentially lower net CO₂ emissions. However, feedstock variability, low yields, and reactor scale‑up remain challenges, and to date these routes contribute only a few percent of total production.
2.6. By‑product Hydrogen
Approximately 18 % of hydrogen arises as a by‑product in chemical processes (e.g., chlor‑alkali, metallurgical operations). This hydrogen can be captured and purified, though volumes are constrained by unrelated market demands.
Each of these traditional methods is mature and widely deployed, but nearly all rely on carbon‑intensive feedstocks. As the push for decarbonization intensifies, low‑carbon alternatives—such as electrolysis powered by renewables (“green” hydrogen) or fossil reforming with CCS (“blue” hydrogen)—are being pursued to replace or retrofit these conventional pathways.
3. Definition of Green Hydrogen and Other Hydrogen Colors
As the hydrogen economy has matured, a “rainbow” of color‑coded labels has emerged to distinguish production pathways by feedstock, process, and associated CO₂ emissions. While no universally agreed color scheme exists, the most commonly used definitions are summarized in Figure 3 [1, 3]:
Green hydrogen: Produced via water electrolysis powered exclusively by renewable energy sources (RES)—for example, wind, solar PV, or hydro. Because the electricity input emits no CO₂ at the point of use, green hydrogen is considered to have zero direct carbon emissions.
Gray hydrogen: Generated from steam methane reforming (SMR) of natural gas without any carbon capture, utilization, and storage (CCUS). This is the dominant industrial route today but carries a high carbon footprint.
Blue hydrogen: Also from SMR (or other fossil routes), but with CO₂ captured and stored or utilized. While blue hydrogen reduces lifecycle emissions, fugitive methane leaks and energy for CCUS mean it still emits significant greenhouse gases.

Turquoise hydrogen: Produced by methane pyrolysis, yielding H₂ and solid carbon. Because no CO₂ is released in the reactor, turquoise hydrogen can be nearly carbon‑free—provided the carbon by‑product is sequestered or valorized.
Beyond these core colors, additional labels have proliferated to capture nuances of feedstock and process:
Orange hydrogen: Electrolysis powered by the average grid mix, whose emissions intensity varies with time and geography. Its carbon footprint depends directly on the grid’s share of RES versus fossil generation.
Pink hydrogen: Electrolysis using nuclear‑generated electricity. While nuclear power itself emits negligible CO₂, the green label is typically reserved for renewables, so nuclear‑powered hydrogen is often distinguished as pink.
Red hydrogen: Hydrogen produced via thermochemical or thermolysis processes (e.g., high‑temperature splitting of water) powered by nuclear or solar thermal energy. Like pink hydrogen, it carries no direct CO₂ emissions.
Yellow hydrogen: Sometimes used interchangeably with orange, but more strictly refers to electrolysis powered by solar PV alone, reflecting the solar “yellow” color.
White hydrogen: Naturally occurring H₂ found in underground deposits, tapped directly without an industrial process. It remains a nascent source, representing a small share of global production.
Brown and Black hydrogen: Produced from lignite (brown coal) or bituminous coal gasification, respectively, without CCUS. These routes are the most carbon‑intensive and are primarily regionally significant where coal is abundant.
This color taxonomy, though imperfect and sometimes overlapping, serves to communicate both the production technology and its environmental footprint at a glance. Green hydrogen stands out as the only pathway with truly zero direct CO₂ emissions, making it the focal point of decarbonization strategies worldwide. At the same time, transitional options—blue and turquoise hydrogen—are gaining attention as means to leverage existing infrastructure and feedstocks while reducing emissions relative to gray routes. Ultimately, consistent definitions and robust certification schemes will be essential to avoid confusion and to ensure that hydrogen labeled “green” or “low‑carbon” truly delivers on its climate promise.
4. Different Electrolyser Types and the Catalysts Used
Electrolysers are a critical component of sustainable hydrogen production, as they convert water into hydrogen and oxygen through electrochemical reactions. Although several electrolyser technologies are available—including alkaline, proton exchange membrane (PEM), and solid oxide electrolysers—the catalysts used within these systems largely determine the overall efficiency and economic viability of the process [2].
In alkaline electrolysers (Figure 4), non-noble metal catalysts such as nickel-based catalysts or transition metal compounds are often preferred due to their high durability in basic media and cost-effectiveness. Nanostructured materials engineered by methods like heterostructural design can enhance active site exposure and improve electron transfer rates. These strategies help offset the lower intrinsic activity of some non-precious metals while maintaining system stability under prolonged operation.

Figure 4. Alkaline electrolyser operation [2]
PEM electrolysers (Figure 5), in contrast, require catalysts that are highly active and robust under acidic conditions. Traditionally, platinum-group metals have been the catalysts of choice owing to their exceptional activity for both the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER). However, given their high cost and scarcity, recent research has focused on novel catalyst architectures—such as single-atom catalysts (SACs) and bimetallic systems—that aim to match or exceed the performance of noble metals with lower material usage. For example, SACs supported on conductive carbon nanomaterials are being developed to maximize the number of active sites and minimize overpotentials, which is crucial for the rapid kinetics needed in PEM systems.

Figure 5. PEM electrolyser operation [2]
Solid oxide electrolysers (Figure 6) operate at higher temperatures and can benefit from catalysts designed using advanced metal oxides and composite materials. These catalysts not only need to sustain high-temperature stability but also deliver efficient ionic and electronic conductivity. Advances in nanostructuring and interface engineering have resulted in catalysts that exhibit improved reaction kinetics and durability under harsh conditions.
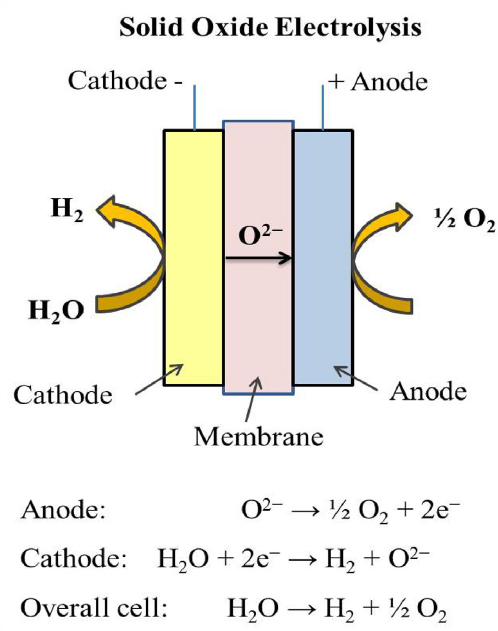
Figure 6. Solid oxide electrolyser operation [2]
Overall, the integration of nanomaterials, innovative synthesis methods, and hybrid catalyst designs is forging new pathways to optimize catalyst performance tailored to each electrolyser type. Such interdisciplinary approaches promise to lower production costs, enhance operational stability, and accelerate the transition to scalable, sustainable hydrogen production.
5. PEM Electrolysers
PEM water electrolysers are a mature and promising technology for the production of high‐purity hydrogen from water using renewable energy sources. In these systems, water is fed into the anode of a cell where it is split into oxygen, protons, and electrons. The solid polymer electrolyte, typically a perfluorosulfonic acid membrane such as Nafion®, plays a pivotal role by transporting protons to the cathode while preventing the mixing of generated gases. At the cathode, the incoming protons recombine with electrons delivered via an external circuit to form hydrogen gas. This mechanism not only ensures an ultra‐pure hydrogen product but also enables compact cell designs with high current densities and fast dynamic response [2, 4].
The thermodynamic efficiency of PEM electrolysis is particularly attractive because the process operates at relatively low temperatures (typically between 20 and 80 °C) compared to other electrolysis technologies. Despite these lower operating temperatures, PEM electrolysers can achieve high energy conversion efficiencies—often in the range of 80–90%—due largely to the excellent proton conductivity of the membranes and the optimized design of the cell components. The concept of thermo-neutral voltage is used to benchmark the minimum energy requirement for the reaction, which, when coupled with effective water management, contributes to the system’s overall efficiency.
One of the distinguishing features of PEM electrolysis is its compact footprint and the ability to operate at high current densities (above 2 A/cm²). These characteristics make PEM systems particularly suitable for integration with intermittent renewable energy sources like solar and wind. However, the high performance comes with challenges related mainly to the cost and durability of the electrocatalysts. For the hydrogen evolution reaction (HER) at the cathode, platinum-based catalysts are generally used because of their unmatched activity and stability in an acidic environment. Similarly, at the anode, catalysts such as IrO₂ and RuO₂ are preferred for the oxygen evolution reaction (OER), even though their high cost is a major barrier to commercial scalability.
In addition to the electrocatalysts, the overall performance and cost efficiency of a PEM electrolyser are heavily influenced by the design of the membrane electrode assembly (MEA), current collectors, and separator plates. The MEA, which comprises both electrodes and the membrane, is critical as it affects proton transport and minimizes ohmic losses. Advanced fabrication techniques, such as catalyst coating on membrane (CCM) methods, are continuously being optimized to reduce the noble metal loading while maintaining efficiency. As research progresses, efforts to develop alternative, less expensive catalyst materials and to improve the durability of the cell components are expected to further enhance the viability of PEM water electrolysis for large-scale commercial hydrogen production.
6. OER Electrocatalysts
Advantages and Problems
Oxygen evolution reaction (OER) electrocatalysts occupy a pivotal role in water-splitting systems for hydrogen production. One of their chief advantages lies in their ability to drive the OER with high catalytic activity, which is essential for achieving efficient water oxidation [5]. Electrocatalysts based on noble metal oxides such as IrO₂ and RuO₂ display superior intrinsic activity due to their favorable electronic structures that facilitate efficient charge transfer and low overpotential requirements. This high conductivity, often associated with overlapping d-orbitals and metal–metal interactions, results in rapid oxygen evolution kinetics under acidic conditions. Moreover, the structural stability of these catalysts under operational environments is an asset; their ability to maintain performance at high current densities is critical for scaling up water electrolysis systems.
Nonetheless, several critical problems also emerge in the deployment of these catalysts. The primary challenge is the high cost and scarcity of noble metals such as ruthenium and iridium. Their limited natural abundance leads to prohibitive costs when aiming for industrial-scale applications, thereby limiting the economic feasibility of water-splitting systems. Furthermore, despite their high activity, many noble metal-based OER catalysts suffer from durability issues (Figure 7). The corrosive operating conditions—especially under acidic environments and high anodic potentials—induce degradation of the catalyst material over time, which compromises long-term performance. Often, the formation of oxide layers or the leaching of active species during prolonged operation can cause a decline in catalytic efficiency.

Figure 7. Lattice oxygen mechanism (LOM) of OER electrocatalysts [5]
There is also complexity in the reaction mechanism at the electrode-electrolyte interface; the dynamics of adsorbed intermediates and the intrinsic sluggish kinetics of the multi-step OER hinder the straightforward optimization of these catalysts. Additionally, many advanced catalysts, while promising in lab-scale experiments, face scalability challenges in synthesis. The precise control over active site dispersion, morphology, and nanostructure—essential factors that define catalytic performance—is often difficult to maintain when transitioning from small-scale prototypes to large-scale production. Finally, the integration of such catalysts into full electrolysis cells is hampered by issues like mass transport limitations and electrode stability, which together challenge the overall system efficiency.
Future Prospects
Looking ahead, the development of next-generation OER electrocatalysts is poised to address many of the current limitations while leveraging the inherent advantages of high catalytic activity. One promising avenue is the design and synthesis of composite materials and heterostructures that combine noble metal oxides with earth-abundant elements. For instance, bimetallic or multi-metallic oxide systems can capitalize on synergistic effects—where an appropriate combination of metals results in an optimized electronic configuration that lowers the overpotential while reducing the noble metal content. Through this approach, it is possible to retain the high activity and conductivity of traditional noble metal catalysts while significantly cutting costs. Researchers are already investigating doped metal oxides and mixed-valence compounds that enhance oxygen mobility and stability under harsh electrochemical conditions.
Another future direction involves the exploration of non-noble metal catalysts. Transition metal compounds such as phosphides, carbides, or nitrides have exhibited promising OER activity and can be tailored using defect engineering and nanostructuring techniques. By introducing vacancies, strain, or heteroatom doping, scientists aim to modify the electronic structure to create new active sites and improve catalytic performance. In addition, developing catalysts with hierarchical architectures—combining micro-, meso-, and nanoporous structures—can enhance reactant accessibility, facilitate efficient mass transport, and improve overall cell kinetics. These nanostructured materials not only promise enhanced activity but also exhibit improved resistance to degradation.
Computational methods, including density functional theory (DFT) and machine-learning algorithms, are poised to accelerate the discovery of novel catalyst formulations by predicting optimal compositions and morphologies before synthesis. Such predictive approaches can narrow down the vast chemical space, guiding experimental efforts more efficiently. Furthermore, integrating advanced in situ characterization techniques, such as operando spectroscopy and high-resolution microscopy, will provide deeper insights into reaction pathways and the evolution of active sites under operating conditions. This knowledge is crucial for fine-tuning catalyst design to achieve the desired balance between activity, stability, and cost-effectiveness.
In the long term, efforts to combine these advanced materials with scalable, green synthesis methods will be instrumental. The development of eco-friendly, cost-effective production protocols will not only reduce the overall carbon footprint of the catalyst fabrication process but also support the transition from laboratory-scale research to commercial implementation. A multidisciplinary approach—merging insights from materials science, electrochemistry, and computational modeling—is essential to unlock the full potential of OER electrocatalysts. By addressing current limitations and optimizing catalyst performance, future research will pave the way for robust, efficient, and sustainable water-splitting technologies that are economically viable for global hydrogen production.
References:
1) A. Ajanovic, M. Sayer, R. Haas, The economics and the environmental benignity of different colors of hydrogen, Int. J. Hydrogen Energy 47 (2022) 24136-24154.
2) S. Shiva Kumar, V. Himabindu, Hydrogen production by PEM water electrolysis – A review, Materials Science for Energy Technologies 2 (2019) 442–454.
3) Jimena Incer-Valverde, Amira Korayem, George Tsatsaronis, Tatiana Morosuk, “Colors” of hydrogen: Definitions and carbon intensity, Energy Conversion and Management 291 (2023) 117294.
4) Niraj Kumar, Radhamanohar Aepuru, Seul-Yi Lee, Soo-Jin Park, Advances in Catalysts for Hydrogen Production: A Comprehensive Review of Materials and Mechanisms, Nanomaterials 2025, 15, 256.
5) Jiangtian Li, Oxygen Evolution Reaction in Energy Conversion and Storage: Design Strategies Under and Beyond the Energy Scaling Relationship, Nano-Micro Lett. (2022) 14:112.
Catalyst Research and Development Laboratory (KARGEL)
Kocaeli University, 2025
Ayşe Nilgün Akın
The Catalyst Research and Development Laboratory (KARGEL) was established to conduct research on catalytic processes in the chemical industry and to develop environmentally friendly catalytic technologies. The laboratory is led by Prof. Dr. Ayşe Nilgün Akın, also, Prof. Dr. Oğuzhan İlgen, Assoc. Prof. Dr. Meltem Yıldız, Assoc. Prof. Dr. Murat Efgan Kibar, Asst. Prof. Dr. Orhan Özcan, and researcher Emel Engintepe are actively engaged in research activities. They are also among the founders of the Alternative Fuel Research and Development Center at Kocaeli University. A key objective of the laboratory is to contribute to the education and training of young engineers with an interest in catalysis. Both undergraduate and graduate students participate in research activities, gaining hands-on experience in catalyst development. The laboratory’s research focuses on the design, synthesis, and characterization of heterogeneous catalysts for various industrial applications

KARGEL maintains strong collaborations with industry, resulting in scientific publications and patents. For example, the patent “A Method for Production of a Catalyst Supporting Material” (WO/2017/048205) and the publication “Optimization, Modeling, and Characterization of Sol-Gel Process Parameters for the Synthesis of Nanostructured Boron-Doped Alumina Catalyst Supports”, conducted in collaboration with TÜPRAŞ as part of a TÜBİTAK project, are significant outputs of these partnerships.
Our collaborations with researchers from other institutions continue to expand. Recent studies include:
• Glycerol as a Feedstock for Chemical Synthesis (10.1002/cben.202400010), in collaboration with TÜBİTAK MAM.
• An Assessment of Boron-Doped Nanotube TiO2 as an Effective Catalyst for CO2 Fixation with Epoxide to Form Cyclic Carbonates (10.1016/j.inoche.2025.113909), in collaboration with Harran University.
• A BAP-KAP project on “Investigation of the Activity and CO Selectivity of Cu-ZrO2-Al2O3 Catalyst in Methanol Steam Reforming by DFT Simulations and Experimental Methods” in collaboration with Gebze Technical University (Assoc. Prof. Dr. M. Oluş Özbek)
• A TUBİTAK 1001 project on “Highly Efficient Synthesis Gas Production via Electrified and Adsorption-Intensified Reverse Water-Gas Shift Reaction” in collaboration with Boğaziçi University (Prof. Dr. Ahmet K. Avcı)
Additionally, undergraduate students contribute to academic research. More than 15 conference proceedings and 3 articles co-authored by undergraduate students were published to date.
• Kibar M:E., Hilal L., Çapa T.B., Bahçıvanlar B., Abdelielil B.B, “Assessment of Homogeneous and Heterogeneous Catalysts in the Transesterification Reaction: A Mini Review”, ChemBioEng Reviews, 10(4), 412-422, 2023
• Çakırca E. E., Tekin G. N., İlgen O., Akın A. N. “Catalytic activity of CaO-based catalyst in transesterification of microalgae oil with methanol” Energy & Environment , 30, 176-187, 2019
• İlgen O., Dincer I., Yildiz M., Alptekin E., Boz N., Çanakçı M., Akın A.N., “Investigation of biodiesel production from canola oil using Mg-Al hydrotalcite catalysts”, Turkish Journal of Chemistry , 31(5),509-514, 2007
Our team actively engages in TÜBİTAK and BAP-funded projects, dedicating substantial effort to the development of sustainable catalytic processes. We possess advanced characterization capabilities, including BET and XRD analyses, enabling rapid evaluation of catalysts. Moreover, our team independently designs and constructs reaction systems within the laboratory.
The primary research areas of our laboratory can be categorized as follows:
1. CO2 Removal and/or Conversion (Boron, graphene and metal doped catalysts, photocatalysts)
2. Energy (Steam reforming, hydrogen and biodiesel production catalysts)
3. Wastewater Treatment (Photocatalytic reaction of dye removal, coloring of TiO2 based catalyst applications, Fenton and photo-Fenton reactions)
4. (Photo) Catalytic Production of Fine Chemicals (Lactic acid synthesis from glycerol)
5. Green Chemistry (Catalysis-related sustainable chemistry topics)
Some selected studies conducted in our laboratory in recent years are given below.
1. Effect of H2S on oxidative steam reforming of biogas for syngas production over MgAl-supported Ni–Ce-based catalysts
This study investigates the effect of hydrogen sulfide (H2S) on the oxidative steam reforming (OSR) of biogas over particulate and monolithic NiCe/MgAl catalysts synthesized via the sol-gel method followed by the incipient wetness impregnation. Catalytic performance was evaluated in a flow reactor at 600 °C, 700 °C, and 800 °C under a constant space velocity of 45,000 mL gcat-1 h-1 and a CH4/CO2/O2/H2O molar feed ratio of 1/0.67/0.1/0.3, under H2S concentrations of 0, 12, and 50 ppm. Characterization was conducted using N2 physisorption, XRD, SEM, TGA, XPS and ICP-OES. Monolithic NiCe/MgAl (NCMA) exhibited reduced carbon deposition across all temperatures, whereas particulate NCMA achieved the highest CH4 (94%) and CO2 (80%) conversions at 800 °C. With 12 ppm H2S, particulate NCMA showed only a 10% decrease in CH4 conversion after 270 min. At 50 ppm H2S, both catalysts experienced significant deactivation, with CH4 conversion declining by approximately 50% after 270 min.
2. Sustainable syngas production by oxy-steam reforming of simulated biogas over NiCe/MgAl hydrotalcite-derived catalysts: Role of support preparation methods on activity
Herein, a series of hydrotalcite-derived MgAl supports were prepared by co-precipitation and hydrothermal methods. NiCe/MgAl catalysts were prepared by loading Ni (10 wt%) and Ce (2.5 wt%) on supports using sequential incipient wetness impregnation and tested in oxy-steam reforming of simulated biogas to produce syngas. N2-physisorption, XRD, XPS, SEM, TEM, ICP-OES, TGA, and Raman were used for catalyst characterization. Reactivity was evaluated at 600 °C, 700 °C, and 800 °C with a constant GHSV (45,000 ml gcat−1 h−1) and feed ratio (CH4/CO2/O2/H2O = 1/0.67/0.1/0.3). The results demonstrate that increasing the co-precipitation aging temperature and the hydrothermal reaction temperature affected the surface area, pore volume, and Ni crystallite size. However, no significant change in reactivity was observed. The NiCe/MgAl catalyst with co-precipitation aging temperature of 140 °C (NCMA140-Co) exhibited lower carbon deposition probably due to higher Ce4+ concentration. Therefore, NCMA140-Co provided high stability and conversions of CH4 (94%) and CO2 (82%) with H2/CO ratio (1.6) at 800 °C without carbon deposition.
3. Kinetic and Parametric Studies on Oleic Acid Esterification Catalyzed by Purolite CT151
In this study, the impact of reaction parameters, reaction kinetics, and mechanism on the esterification of oleic acid with methanol using Purolite CT151 as a heterogeneous catalyst was investigated. The effects of molar ratio, reaction time, and catalyst amount were examined. The highest oleic acid conversion of 84 % was achieved under the following conditions: methanol/oleic acid molar ratio of 12:1, 20 wt. % catalyst amounts, a reaction time of 7 h, and a reaction temperature of 67 degrees C. The surface characterization was performed with FTIR and scanning electron microscope analysis. The proposed reaction model was based on the Eley-Rideal mechanism, where methanol adsorbed onto the catalyst surface reacted with oleic acid before water desorption. The Purolite CT151 catalyst used in the esterification of oleic acid could be a potential catalyst for waste cooking oils with high FFA content. The impact of reaction parameters, reaction kinetics and mechanism on the esterification of oleic acid with methanol using Purolite CT151 as a heterogeneous catalyst was investigated. The effects of molar ratio, reaction time, and catalyst amount were examined. Eley-Rideal mechanism was proposed as potential reaction mechanism. The kinetic data were also well matched with the Eley-Rideal mechanism where the surface reaction was assumed as the rate limiting step. The results demonstrated a high level of agreement between the experimental and calculated reaction rates. Thus, the use of Purolite CT151 in the esterification of oleic acid is considered a promising approach for processing waste cooking oils with high FFA content. Future studies could further explore the application of this catalyst in esterification and transesterification reactions involving such waste cooking oils.
4. Synthesis of the Environmentally Friendly Fuel Bioadditive Solketal by the Green Catalytic Membrane PVA/PAMPS
Solketal is produced using glycerin and acetone with the green catalytic membrane as a heterogeneous catalyst. Solketal is a bio-additive that improves combustion efficiency and fuel properties to control air pollution. The catalytic membrane was created using polyvinyl alcohol (PVA) green polymer, poly (2-acrylamido-2-methyl-1-propanesulfonic acid) (PAMPS) homopolymer, and sulfosuccinic acid (SSA) as a crosslinking agent. By catalyzing the reaction and absorbing water by-product, the catalytic membrane increased the yield. The experiments were conducted in a batch reactor, and the impact of various factors, such as the amount of catalyst, reaction temperature, and the initial molar ratio of reactants, on the reaction yield was evaluated. The optimal solketal yield of 95% was obtained after 3 hours of reaction time, with a reaction temperature of 60 degrees C, an acetone/glycerin molar ratio of 6/1, and a PVA/PAMPS catalytic membrane ratio of 70/30. The catalytic membrane's capacity to absorb water had a favorable impact on the reaction yield, which could be used to reduce air pollution. Sorption enhanced eco-friendly production of solketal by the catalytic membrane is a promising alternative method to the methods that use other catalysts.
5. Glycerol as a Feedstock for Chemical Synthesis
Glycerol, defined simply as a colorless, sweet syrupy liquid extracted from fatty substances through saponification, is an alcohol with three hydroxyl (OH-) groups in its structure. Glycerol has many uses in the consumer market. It is used primarily in personal care products, as an adhesive and sealing agent and many applications. Glycerol, whose name is propane-1,2,3-triol, standardized by the International Union of Pure and Applied Chemistry (IUPAC), CHO open formula CH2OH-CHOH-CH2OH. It can be said that glycerol, a by-product of biodiesel, is produced in very high quantities. Retention of the produced glycerol will lead to cost increases and environmental problems that may directly affect the development of the biodiesel market. Due to the supply of glycerol to the market in large quantities, glycerol prices have hit the bottom, and therefore, the income and profitability of biodiesel production factories from the sale of glycerol have decreased. This situation clearly shows that the excess of glycerol now poses an obstacle to developing the biodiesel market. This article aims to list the valuable chemicals into which glycerol, produced in large quantities as a biodiesel by-product, can be converted under a single heading and to detail the studies carried out on this subject. The conversion of glycerol into higher value products can enhance the economic viability of biodiesel production and other industries that produce glycerol as by-product. This can help in offsetting the costs and increasing the profitability of these industries. This review summarizes the uses of glycerol at different industries and highlights the importance of these areas.
6. Effects of different catalysts on the mechanical, thermal, and rheological properties of poly(lactic acid)/polycarbonate blend
Poly(lactic acid)/polycarbonate (PLA/PC) blends are often explored for use in durable applications such as mobile phones, laptops, and automotive parts. With the use of this blend, while reducing the dependence on the petroleum-based polymers, environmental pollution can be prevented. However, PLA/PC blend is immiscible and needs to be compatibilized. To encourage compatibilization and improve of the performance of the PLA/PC blend, TiO2 and CeO2 were incorporated into the blend. The effects of catalysts type and amount on the structural (Fourier transform infrared analysis-FTIR), morphological (scanning electron microscopy-SEM), rheological, mechanical, and thermal properties (thermogravimetric analysis-TGA, differential scanning calorimeter-DSC) of the PLA/PC blends were evaluated in this study. FTIR results revealed that the catalysts promoted the reaction between PLA and PC. The modulus of the blend increased with the addition of catalyst. The CeO2 containing blends exhibited brittle behavior which was also supported by SEM micrographs. The added catalysts acted as a lubricant, lowered the complex viscosity of the blend, and made processing easier. With the addition of fillers at all amounts, thermal decomposition temperature decreased while the residual weight at 800 °C increased with the inclusion of 3 wt% CeO2. Mechanical results revealed that the highest tensile strength and elongation values were obtained for 0.5 wt% CeO2 and 0.5 wt% TiO2, respectively. It was observed that the loading level and type of catalyst significantly affected the PLA/PC blends mechanical and thermal properties.
7. An assessment of boron-doped nanotube TiO2 as effective catalysts for CO2 fixation with epoxide to form cyclic carbonates
One of the key methods for CO2 fixation in the applied chemical and industrial sectors is the conversion of CO2 into cyclic carbonates, which is significant from the standpoint of green chemistry and reaching net zero emissions. In this context, a series of novel boron-doped nanotube TiO2 compounds (B1-ntTiO2 and B16-ntTiO2) have been synthesized as the catalysts for the synthesis of cyclic organic carbonates via CO2 insertion various epoxides under suitable conditions. The characteristic properties of the catalysts were examined by XRD, FT-IR spectra TEM and SEM. Subsequently, under suitable conditions (1.6 MPa, 100 °C, 2 h), novel boron-doped nanotube TiO2 compounds were tested for the coupling reaction of CO2 with various epoxides to obtain target cyclic carbonates for carbon neutrality and as a substitute for toxic reagents like phosgene. There was an attractive increase in efficiency with the use of boron-doped catalyst, and 96 % efficiency and 99.2 % selectivity were achieved with the B16-ntTiO2 catalyst. Reusability tests exhibited that the efficiency of the catalyst decreased to 80 % on the third cycle, the selectivity was found to be around 99 %.
8. Case analysis and future aspects of photo/thermocatalytic hydrogen production from methanol
The need for alternative energy is growing daily due to population growth and fuel consumption around the world. Hydrogen, as one of the promising alternative energy sources, is on the agenda worldwide. Methanol is a chemical compound that is rich in hydrogen. In this review, methanol decomposition reactions, such as photocatalytic, photothermal and thermal reactions, are examined and predictive results are discussed. The properties of an active catalyst and reaction conditions were studied.
9. Methanol steam reforming kinetics using a commercial CuO/ZnO/Al2O3 catalyst: Simulation of a reformer integrated with HT-PEMFC system
This study provides a kinetic examination of methanol steam reforming (MSR) over a Cu- based commercial catalyst (CuO/ZnO/Al2O3, Alfa Aesar) as a function of CH3OH and H2O partial pressures at 246 ℃ and 1 atm in a once-through flow reactor. A power rate law was used to best describe the experimental rate data by linear and non-linear regressions at the operating conditions where transport bottlenecks were eliminated. Comparison of the rate parameters indicated that a strong correlation was suggested by non-linear regression giving reaction orders of 0.29 for methanol and 0.09 for water along with a frequency factor of 53.48 (molCH3OH s-1 gcatalyst-1 kPa-0.38) and an activation energy of 65.59 kJ mol-1. A simulation study of the rate equation to analyze an integrated system of a reformer and an HT-PEMFC was also conducted. The results demonstrate that the system has the potential to produce 15 W power output.
10. Thermodynamic modelling and optimization of oxy-reforming and oxy-steam reforming of biogas by RSM
In this study, a comparative thermodynamic equilibrium calculation of biogas oxy-reforming and oxy-steam reforming processes to produce syngas has been conducted by Aspen Plus simulation software. The effects of temperature (600–800 °C), pressure (1−20 atm), and inlet O2/CH4 (0−1.0), H2O/CH4 (0−3.0), and CO2/CH4 (0.3−1.0) mole ratios on the equilibrium compositions of products were determined. The operation of the process was optimized using Gibbs free energy minimization method and statistical approach: response surface methodology (RSM). Optimum operating conditions CH4/CO2/O2 = 1:0.51:0.12 at 788 °C and 1 atm for oxy-reforming and CH4/CO2/H2O/O2 = 1:0.63:0.19:0.07 at 780 °C and 1 atm for oxy-steam reforming were obtained to reach maximum H2 yield, CH4 and CO2 conversions by minimizing carbon selectivity to produce syngas for methanol production.
Please visit our website:
https://avesis.kocaeli.edu.tr/arastirma-grubu/kargel
Contact:
• katalizor@kocaeli.edu.tr
• akinn@kocaeli.edu.tr
• +90 262 303 3548
PhD Stories
By PhD. Candidate Mert Özden
Hello, I am Mert Özden, a PhD candidate in the Chemical Engineering Department at Boğaziçi University. My journey began in 2017 as a master’s student, evolved into a PhD pursuit in 2019, and since 2022, I’ve been working as a teaching assistant. Throughout this time, I’ve had the privilege of working under the steadfast support and trust of Prof. Dr. Ahmet Kerim Avcı within the Sustainable Process Intensification, Catalysis, and Reaction Engineering (SPICE) group. My PhD thesis explores the process intensification strategies for direct conversion of carbon dioxide to dimethyl ether (DME).
For any graduate student, the greatest pillars of support are family, colleagues, and their thesis advisor. These are the people who patiently listen to the trials and tribulations of the research process. While a gas chromatograph might be a silent companion in the lab, it’s not exactly forthcoming with advice. I owe heartfelt thanks to my wonderful wife and life partner, Yağmur, who has shared my burdens; to my parents, whose unwavering material and emotional support has never faltered; to Semih, my partner in lab for eight years; and to my advisor, Prof. Dr. Ahmet Kerim Avcı, whose boundless patience, wisdom, and kindness have been a steady source of strength. Your encouragement has made this challenging journey so much more bearable.
As an eager young researcher fresh from completing my master’s degree, I didn’t hesitate to dive into a PhD. Yet, as with many paths, it’s hard to foresee the difficulties that lie ahead when you first start out. The pandemic, which struck just as I was wrapping up coursework and beginning my thesis research, was undoubtedly the toughest hurdle. For us experimental researchers—wrench in hand, unable to walk away from our labs—the separation brought by the pandemic hit especially hard. With special permission, we returned to the lab after a year-long hiatus, working in shifts to reduce infection risks. The strangest part? Conducting experiments while the bustling streets of Istanbul, the region’s most populous city, stood eerily silent. I doubt I’ll ever see it that quiet again.
This PhD journey has opened unexpected doors, nurtured my growth as a curious and inquisitive individual, and connected me with people whose friendship I truly cherish. One standout experience was my trip to SESAME in Jordan, made possible by the support of Prof. Dr. Emrah Özensoy and Dr. Kerem Emre Ercan. Working at the SESAME synchrotron—a Middle Eastern facility where Turkey is a partner—not only sharpened my skills in advanced characterization techniques but also gave me the chance to explore a country I might not have otherwise visited. This opportunity underscored the immense value of collaborating with other institutions and research groups in academia.
Looking forward, my post-PhD aspiration is to join an academic institution or R&D organization that fuels my enthusiasm for catalysis and process design and development. Contributing to a field I believe is vital for our world’s future—both through scientific literature and societal impact—is a driving force for me. Even after eight years in this field, I still find immense joy in reading a fresh publication or tackling a student’s thought-provoking question and researching the answer together.
As I close, I want to express my deep gratitude to the Turkish Catalysis Society and Prof. Dr. Ayşe Nilgün Akın for inspiring me to share my story. The presentations I’ve delivered and the people I’ve met through society’s events have spurred me to work harder in this field and enriched my learning and growth. These events offer invaluable opportunities for me and fellow students alike. It’s been an honor to contribute to this vibrant community since my first catalysis school. I look forward to seeing you all in Sivas.
Recent Selected Papers in Our Catalysis Community
In recent months, there have been exciting research studies in catalysis research in Turkey. Here are the short summaries:
Machine Learning
Kilic, A., Uzun, A., Yildirim, R., Eroglu, D. (2025). Ionic liquid electrolytes for metal-air batteries: High-throughput screening and machine learning modeling. Electrochimica Acta, 524 (2025) 145997.
This work aims to develop efficient electrolyte systems for metal-air batteries by using machine learning to predict gas solubilities in ionic liquids (ILs). By employing COSMO-RS calculations and structural descriptors, random forest models are built to evaluate IL properties and screen promising candidates. The study identifies 139 ILs out of 31,080 as suitable based on criteria like low melting point, low viscosity, and high oxygen solubility and selectivity.
Balde, M.S., Karakış, R., Ateş, A. (2025). Estimation of specific surface area and higher heating value of biochar and activated carbon produced by pyrolysis and physico‑chemically assisted pyrolysis of biomass using an artificial neural network (ANN). Biomass Conversion and Biorefinery, https://doi.org/10.1007/s13399-025-06728-w.
This study investigates how physical and chemical activation of biomass, specifically tea waste and hazelnut shells, influences the properties of biochar and activated carbon produced through pyrolysis at varying temperatures. Machine learning models, especially artificial neural networks (ANNs), were developed and found to effectively predict the higher heating value (HHV) and specific surface area (SSA) of biochar, with the resilient backpropagation algorithm delivering the best performance. Thermodynamic analysis showed that activation conditions and temperature significantly impact the energy and exergy yields, with activated carbon achieving higher energy efficiency than conventional biochar.
Sustainable Materials
Turhan, E.A., Yarıcı, T., Dizman, B., Binay, N., Bengu, B., Erkey, C., Senses, E. (2025). Fiber level catalyst-free oxidative carboxylation enhances physical properties of wood polymer composites. Polymer Composite, DOI: 10.1002/pc.29690.
This study introduces a one-step, catalyst-free nitric acid steam oxidation method to modify wood fibers for use in medium-density fiberboard (MDF), aiming to enhance performance while addressing environmental and health concerns associated with traditional treatments. The modification significantly improved the mechanical properties and dimensional stability of MDF panels, increasing internal bond strength by 3.3 times and reducing water uptake by nearly 90%. Additionally, the process lowered adhesive curing temperature by 50 °C and reduced formaldehyde emissions, offering energy savings and improved environmental compliance.
Electrocatalysis
Alsuhile, A., Pein, P.S., Barım, S.B., Bozbağ, S.E., Smirnova, I., Erkey, C., Schroeter, B. (2025). Synthesis of Pt Carbon Aerogel Electrocatalysts with Multiscale Porosity Derived from Cellulose and Chitosan Biopolymer Aerogels via Supercritical Deposition for Hydrogen Evolution Reaction. Adv. Energy Sustainability Res. 2025, 2400433.
This study evaluates carbon aerogel-supported platinum electrocatalysts for hydrogen evolution, comparing their performance and stability to conventional carbon black-based systems. The self-synthesized aerogels, derived from cellulose and chitosan, demonstrated similar electrocatalytic activity to commercial catalysts despite significantly lower platinum loading. Notably, these aerogel-based catalysts exhibited much higher durability, with minimal performance degradation over time, highlighting their potential for efficient and sustainable hydrogen production.
Photovoltaics & Surface Engineering
Gümüş Çiftci, B., Güldür, Ç., Güneş, S. (2025). Inverted Pyramid Texturization of Monocrystalline Silicon Surface by Cu-Assisted Chemical Etching at Different Conditions. Silicon, https://doi.org/10.1007/s12633-025-03278-8.
This study demonstrates that inverted pyramid (IP) structures on silicon surfaces, created using a single-step Cu-assisted chemical etching method, effectively reduce surface reflectivity and enhance light trapping in solar cells. Optimizing HF concentration and etching duration resulted in a reflectivity as low as 2.69% after passivation, significantly outperforming traditional upright pyramid textures. The optimized IP-textured silicon achieved a solar cell efficiency of 22.24% and a short-circuit current of 10.12 A, highlighting its potential for improving monocrystalline silicon solar cell performance.
Process Simulation
Uysal, D.S., Kalıpçılar, H., Karakaş, G. (2025). Kinetic Analysis and Simulation of Dicyclopentadiene/ Cyclopentadiene Production by Using Reactive Batch Distillation of Pyrolysis Gasoline. Energy&Fuels, https://doi.org/10.1021/acs.energyfuels.5c00569.
This study explores the use of reactive batch distillation to produce cyclopentadiene by cracking dicyclopentadiene in pyrolysis gasoline. Reaction kinetics and vapor–liquid equilibrium data were experimentally determined and used to simulate the process in Aspen Plus, analyzing key operational parameters. The simulation showed that cyclopentadiene with 90 wt% purity and a 76.5% overall yield can be achieved in two steps using a single batch distillation column under optimized conditions.
Methane Activation
Özdemir, H., Çifçioğlu, E., Öksüzömer, M.A.F. (2025). Enhanced oxidative coupling of methane using strontium and barium doped lanthanum or samarium oxide nanocatalysts. Chemical engineering communications, https://doi.org/10.1080/00986445.2025.2481515.
This study investigated La₂O₃ and Sm₂O₃ nanoparticle catalysts doped with varying amounts of Sr or Ba for the oxidative coupling of methane (OCM). Sr-doped catalysts, particularly 8 wt% Sr/La₂O₃, showed superior performance with a maximum C₂ hydrocarbon yield of 14.3% at 510 °C, due to better Sr diffusion and enhanced surface basicity. In contrast, Ba had minimal effect, and while high surface area lowered performance, increased basicity improved catalytic efficiency and long-term stability.


Previous issue answers Newsletter #12:
1. Photocatalysis, 2. Selectivity, 3. SOLCAT, 4. Electrochemical, 5. Machine Learning, 6. Neural Network, 7. ANN, 8. Supervised Learning,
Announcements


In 2025, there will be a highly active program of events in the field of catalysis.
• From June 25 to 28, 10th National Catalysis Conference (NCC10), organized by the Catalysis Society and Cumhuriyet University, will take place in Sivas. We look forward to welcoming you there.
• From July 9 to 11, The 14th International Symposium of the Romanian Catalysis Society (RomCat 2025), organized by Societatea De Cataliza Din Romania will be held on Gluj-Napoca, Romania. For more information: http://www.unibuc.ro/romcat/
• From August 31 to September 5, 16th Europacat Conference, organized by EFCATS, of which our society is a member, will be held in Trondheim, Norway.
• From September 1 to 4, 36th National Chemistry Congress, organized by Van Yüzüncü Yıl University will feature a "Catalysis" session supported by Catalysis society."
• From September 9 to 12, the 16th National Chemical Engineering Congress, organized by Bolu Abant İzzet Baysal University, will host a special session on "Catalysis and Reaction Engineering," supported by Catalysis society.

