Dear Catalysis Researchers,
Welcome to our monthly newsletter Magic Powder dedicated to the catalysis research and development.
In this monthly issue, we present an article about Catalysis Research Across Electromagnetic Spectrum in CACTUS ZONE from our very valuable founding president Prof. Dr. Deniz Üner (Page 2) In addition, you can see short summaries of most recent high impact research articles conducted by Turkish Catalysis Community (Page 4).
Thank you for being part of our catalysis community. We look forward to bringing you more exciting updates in the next edition of our newsletter. We are always open to contributions of academic and industrial partners in our upcoming issues. We would also like to state that we would be happy to see you at the ASC7 and 35 th national chemistry congress and look forward to your participation by sending an abstract.
In this monthly issue we did not forget to challenge you with our puzzle from Professor Merlin Catalystorius 😊.
Editorial Board:
Prof. Dr. Ayşe Nilgün AKIN
Prof. Dr. N. Alper TAPAN
Dr. Merve Doğan Özcan
Contact info:
Email:katalizdernegi@gmail.com
Linkedin: https://www.linkedin.com/in/kataliz-derneği-272879a
CATALYSIS RESEARCH ACROSS THE ELECTROMAGNETIC SPECTRUM
UNER LABS AT ODTU a.k.a THE CACTUS ZONE
The catalysis research conducted at Uner labs can be viewed from different perspectives. Below you will find a brief summary. The core strategy of our lab is to be able to answer the why question, and design the next component whether it is the catalyst itself or the reactor/process around it. In most of the situations, the quest for finding a better catalyst has to be stopped at some feasible point due to the limitations imposed by the nature. Being able to know this limit is very important. Because the room for improvement becomes the process, with its infinite possibilities. But we have to make sure that we looked at our catalyst from all directions using all possible analytical techniques.

The work we do must be relevant to the status quo. We must be observant to the problems around us and be able to offer solution strategies with our research. Our research outputs have the potential to make large scale impact, especially through the training it provides for the new generation of researchers. Of the possible routes that could shape the research here, our major thrust is problems relevant to sustainability. Sustainability concept can be interpreted in many different dimensions. Here I will address those that matters most to me. The sustainability in my research strategy includes the sustainability of the ecosystem that we are living in. As a result of this priority, the research questions that I tackle involves water, air and energy. The sustainability of research itself is also very important, especially in fluctuating economic backgrounds.
Your research is sustainable, if you can continue with your research, regardless of the hindrances.
To be able to do research in a sustainable framework is very important. This strategy requires self sufficiency, understanding the research equipment, its working principles and be able to modify and improve the quality of the measurement. Developing new ways of using the equipment is embedded in this strategy. We set up our own equipment to do the best of our abilities. This gives us the power to maintain the equipment. We receive this power to the excellent manufacturing infrastructure that is offered to us by the technical staff at our university and in our vicinity. I am very proud to see some graduates of our lab and our department successfully flourishing in the risky trenches of innovation and entrepreneurship. Although it somehow counterargues the research quality measured around the funding needed, by using the basic building blocks such as some resistive wires, a small temperature controller and a thermocouple, members of my group can build a heating element that fits our needs. This is a luxury that can not be bought with any money.
Currently we raised our prospects to the sky, and harnessing the solar energy with the help of mirrors that can be bought off the shelf. If we have some flow controllers, tubing and connectors, we can construct our gas manifolds, prepare gas mixtures to test our catalysts. If we are given a vacuum pump and a pressure measurement device, we can construct and use static/or dynamic adsorption units. The critical components we need are analytical instruments to determine the composition of gases at the inlet and exit of the reactors. My recommendation to any striving academic researcher or graduate student is to gain a basic level of skills of components and their intricate principles of working to be able to construct and troubleshoot their equipment. Instead of having the best lab or best equipment, I focused on the capacity to do cutting edge research within the limitations, while striving for the excellence within. When playing in the limitations of research game, we would gain depth of understanding when tackling with problems.
Admitting that you do not know is not weakness, it is an opportunity to grow!
Depth of understanding is another important core principle, and paradoxically it comes with the simple honest statement of “I don’t understand this”. This statement leads us either to throw in the towel and take some answer axiomatically and move forward, or to sink into the problem with our teeth and nails if needed. It is the second route that trained me and the graduate students that passed through the painful trenches of the “CACTUS zone”. This frontier culture still builds an amazing strength and stamina within the students and graduates.
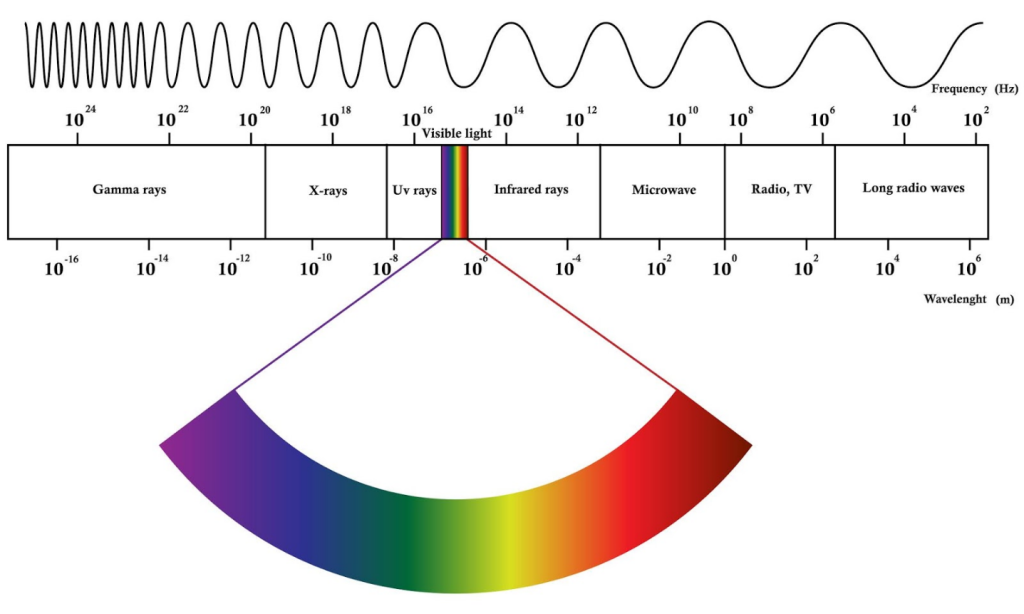
Harnessing the power of the electromagnetic radiation is the third dimension, and it is all what we are. In Uner labs, we aim for the capacity to be able to access as broad spectrum of frequencies as possible. Below, I will explain what each method can measure, and how we use the method to explore the deep mysteries of heterogeneous catalytic conversions.
The lowest wavelength that is used in our labs falls in the range of infrared spectrum: in the region of micrometers. Infrared spectrum is in the realm of the thermal energy and its distribution across molecules and the vibrations within. In the infinite network of molecular bonds, interaction of materials with a proper wavelength of the electromagnetic spectrum can lead to absorption of photons. The absence of these photons at a particular frequency, measured through sensors and detectors, indicate absorption of these photons in a vibrational state, exactly at that frequency. By this means, one can identify a compound or the presence of a functional group in a molecule. By monitoring the formation or disappearance of these functional groups during chemical conversions, we infer conclusions about the quality or specificity of our catalysts. We do this by Diffuse Reflectance Infrared Spectroscopy (DRIFTS). The method is semi quantitative as the spectrum does not allow us compare the intensities to understand the real amounts of different vibrations. But we can measure the changes within one species and relate that to its amount. As such, this method can only allow us to make quantitative analysis, if our measurements are supported by another method that provides quantitative information.
Microwaves are longer wavelengths in the region of centimeters. Interaction of microwaves allow us to identify the unpaired electrons in a catalyst. The catalytic action, if succinctly summarized, is the give and take of electrons. As such the identification of free electrons, or their absence, can reveal a lot about a catalyst and its activity. We use a benchtop Electron Paramagnetic Resonance (EPR) or Electron Spin Resonance (ESR) spectrometer to characterize the catalysts and to monitor the catalytic processes. The ESR method relies on a quantum mechanical phenomenon called Zeeman splitting. Electrons have degenerate energy states, i.e. the energy of the electrons in the spin up and spin down states have the same energy levels in the absence of a magnetic field. When they are in a magnetic field, the spin up and spin down states of electrons are separated by an energy level difference dictated by the magnetic field strength and Bohr’ magneton. Using pulses of electromagnetic radiation with microwave frequency, it is possible to reorient these states relative to the magnetic field, and monitor their relaxation through which we can infer the number, and frequency of these spin states. The method allows us to monitor electrons generated through photons during photocatalysis, and the presence of unpaired electrons in a catalytic framework.
Radio Waves are even longer wavelengths in the region of meters. We use them in Nuclear Magnetic Resonance (NMR) Spectroscopy. NMR spectroscopy relies on the Zeeman splitting phenomena observed in nuclear spins. Unpaired protons in the nucleus have degenerate energy levels in the absence of a magnetic field. When they are in a magnetic field, they are separated by an energy level that is proportional to the magnetic field strength and the gyromagnetic ratio of the nucleus in question. NMR spectroscopy is the unique jewel in our box due to the wide array of studies it enables us. The NMR spectroscopy enables us to monitor the spin dynamics. The spin dynamics can tell us a lot about how the atoms in a molecule interact with the other atoms, especially those with an NMR signal. Coupling between the spins and their motional averaging strategies can give us quantitative exchange rate information, or a boundary of that rate as it may be larger than or smaller than… statement. We can learn about the molecular structure, including the stoichiometry of the atoms. Multidimensional NMR spectroscopy allow us to elucidate the connectivity within a molecule, including the dihedral angles. The timescales of the events that one can by NMR spectroscopy is very wide, especially when multi pulse sequences are used for selective excitation experiments. The method has a very significant shortcoming as the signals are very weak, as a result, noise averaging is needed especially when monitoring the adsorbed molecules. What is unique to our lab is that we have a close coupled gas manifold to our low field benchtop NMR spectrometer. The close coupling removes many of the hydrodynamic barriers one would experience with a high field NMR spectrometer, and the data we measure is free of transport limitations imposed by the necessities of maintaining a superconducting field.
Ultraviolet radiation is below the violet part of the spectrum, it is not visible to our eyes. By measuring the absorbance of ultraviolet spectrum, we can identify the presence and concentration of molecules and identify their concentrations. This method comes in very handy when liquid phase quantitative analysis is needed.
Although direct electromagnetic interaction is not involved, two staple measurements help us even further characterize our catalysts. One of them is Temperature Programmed Reduction/Oxidation. This is critical for us to determine the reducibility of our catalysts. The reduction temperatures and the amount of reducing agent that is consumed can give us in depth information about the oxidation state of the bulk of our catalysts. In some cases, surface events can also be distinguishable. This information is extremely valuable, especially when the catalytic cycle is of Mars van Krevelen type. In other words, the catalyst changes its oxidation state during the initial phase of the cycle and recovers the original oxidation state at the end of it.
Our lab is equipped with reaction systems coupled to chemical analysis units such as gas chromatographs, home build gas analyzers and a residual gas analyzer, formerly known as a mass spectrometer. These systems enable us to monitor the catalytic activity under close to the realistic conditions and elucidate the rate laws that are duly needed to design a scaled-up reactor system. Feel free to contact me at uner@metu.edu.tr for your questions, and have an opportunity to visit the CACTUS ZONE for short, or longer, more committed periods.
Recent Selected Papers in our Catalysis Community
In recent months, there have been exciting research studies in catalysis research in Turkey. Here are the short summaries:
Computational Catalysis
Ozkan, D. M., Uzun, A., Caglayan, B. S., & Aksoylu, A. E. (2023). A DFT study on the role of oxygen vacancy on m-ZrO2 (1¯ 11) in adsorption and dissociation of CO2. Surface Science, 736, 122336.
The stability of Catalytic Dry Reforming of Methane (CDRM) catalysts depends on their resistance to coke formation, achieved by removing carbon from hydrogen production sites through mobile surface oxygen generated by CO2 dissociation. This study investigates the role of oxygen vacancies in activating and dissociating CO2 molecules on m-ZrO2 surfaces, commonly used as CDRM catalyst supports. DFT calculations reveal that nonstoichiometric m-ZrO2 surfaces with oxygen vacancies facilitate CO2 dissociation, indicating their potential in practical CDRM catalyst applications.
Desulfurization catalysts
Bulut, B., Atakül, H., Aksoylu, A. E., & Tantekin-Ersolmaz, Ş. B. (2024). Deep adsorptive desulfurization of liquefied petroleum gas over Cu ion-exchanged Y zeolite. Separation and Purification Technology, 338, 126431.
Deep desulfurization of fuels is crucial for reducing sulfur oxides (SOx) emissions, which contribute to smog, global warming, and acid rain during combustion. Liquefied petroleum gas (LPG) is a promising low-emission fuel, but its sulfur content varies depending on the source. A copper-modified Y zeolite (Cu-Y) adsorbent was developed for removing dimethyl disulfide (DMDS) and thiophene (TP) from LPG, exhibiting significantly improved adsorption performance compared to the original NaY zeolite, particularly with Cu-Y loaded at 7 wt% Cu2+ and calcined at 550 ◦C. Characterization and regeneration studies demonstrate the effectiveness and durability of Cu-Y zeolite for practical applications, maintaining high adsorption capacity over multiple cycles.
Environmental catalysts
Kaya-Özkiper, K., Uzun, A., & Soyer-Uzun, S. (2024). Boosting methylene blue adsorption capacity of an industrial waste-based geopolymer by depositing graphitic carbon nitride onto its surface: Towards sustainable materials for wastewater treatment. Chemical Engineering Science, 284, 119398.
Surface modification of a geopolymer derived from red mud (RM) and metakaolin (MK) was achieved by depositing urea-derived graphitic carbon nitride (g-C3N4), resulting in g-C3N4/RM-MK-GP with exceptional methylene blue (MB) adsorption capacity of 170.9 mg g−1, surpassing both GP and g-C3N4 alone. Kinetic studies indicated chemisorption as a significant factor in the adsorption process, while regenerability was demonstrated over four consecutive cycles. Characterization revealed that the combination of surface functional groups from g-C3N4, RM-MK-GP's hydroxyl and silanol groups, and the geopolymeric framework's exchangeable charge balancing cations contributes to the unique adsorption capabilities of g-C3N4/RM-MK-GP, presenting a sustainable and cost-effective adsorbent for wastewater treatment.
Hydrogenation catalysts
Yalcin, K., Kurtoğlu-Öztulum, S. F., Sarac Oztuna, F. E., Kanat, G. H., Unal, U., & Uzun, A. (2024). Active Sites and Their Individual Turnover Frequencies for Ethylene Hydrogenation on Reduced Graphene Aerogel. Langmuir.
Different reduced graphene aerogels (rGAs) were prepared by varying thermal reduction temperatures, affecting surface area and density of defect sites. The rGA reduced in Ar at 900°C exhibited the highest catalytic performance for ethylene hydrogenation. Analysis of individual turnover frequencies (TOFs) revealed that polyene-like structures are more active than amorphous carbon defects, offering insights for the rational design of graphene aerogel-based carbocatalysts.
Metal organic frameworks
Kümbetlioğlu, F., Oskay, K. O., & Ateş, A. (2024). Preparation and characterization of Fe3O4/ZIF-8 and Fe3O4–MnO2/ZIF-8 composites. Journal of the Iranian Chemical Society, 1-10.
Metal-organic frameworks (MOFs), particularly zeolitic imidazole frameworks (ZIF-8), are valued for their stability and porosity, despite limitations in activity and reusability. To address these issues, Fe3O4 and Fe3O4–MnO2 were incorporated into ZIF-8, maintaining its polyhedral structure while enhancing conductivity. Analysis revealed reduced surface area and mesopore formation, with MnO2/ZIF-8 exhibiting the highest specific capacitance in supercapacitor applications, indicating potential in diverse areas such as gas storage, adsorption, catalysts, and membranes.
Reforming catalysts
Sarıyer, M., Sezgi, N. A., & Doğu, T. (2024). Process intensification methods in steam reforming of ethanol with nickel impregnated mesoporous carbon: Microwave heating and sorption enhanced reforming. International Journal of Hydrogen Energy.
The study focused on producing hydrogen-rich gas for fuel cell-powered cars through steam reforming and sorption-enhanced steam reforming of ethanol using a nickel-impregnated mesoporous carbon catalyst. It compared conventional and microwave-heated reactor systems, finding that microwave heating resulted in higher hydrogen yield and purity, exceeding 97% in the sorption-enhanced steam reforming process at 500°C without carbon monoxide and dioxide. Both process intensification methods, sorption-enhanced reforming and microwave heating, demonstrated potential for a sustainable and energy-efficient hydrogen production system.
Photocatalysis
Bouziani, A., Yahya, M., Bianchi, C. L., Falletta, E., & Celik, G. (2023). Ternary polyaniline@ Bi2O3-BiOCl nanocomposites as innovative highly active photocatalysts for the removal of the dye under solar light irradiation. Nanomaterials, 13(4), 713.
Ternary PANI@Bi2O3-BiOCl nanocomposites were synthesized and thoroughly characterized using various analytical techniques. The interaction between PANI and semiconductor components facilitated efficient separation and transfer of photogenerated charge carriers, enhancing the photocatalytic degradation of methylene blue under solar light. The 0.5%PANI@Bi2O3-BiOCl composite exhibited the best performance, achieving 80% methylene blue degradation in 2 hours, four times higher than pristine Bi2O3-BiOCl, and maintained stable activity over four cycles, with scavenger studies providing insights into the photocatalytic mechanism.
CO2 electroreduction catalysts
Yusufoğlu, M., Tafazoli, S., Jahangiri, H., Yağcı, M. B., Balkan, T., & Kaya, S. (2024). ALD-Engineered Cu x O Overlayers Transform ZnO Nanorods for Selective Production of CO in Electrochemical CO2 Reduction. ACS Applied Materials & Interfaces.
The electrochemical CO2 reduction reaction (CO2RR) is a promising approach for reducing atmospheric CO2 levels and generating clean energy, with a focus on CO production due to its industrial viability. CuZn-based electrocatalysts offer a cost-effective alternative to conventional CO-selective catalysts, but their widespread use requires a deeper understanding of their performance. Through electrodeposition of ZnO nanorods and atomic layer deposition (ALD) of CuxO overlayers, significant enhancements in CO selectivity, electrochemical surface area (ESCA), and introduction of active sites in the ε-CuZn4 phase were achieved, suggesting the crucial role of CuxO in stabilizing ZnO during CO2RR.
Upcoming Catalysis Events
7th Anatolian School of Catalysis (Date: 01-05/09/2024)
Sponsored by European Federation of Catalysis (EFCATS)!!
Web site: https://meetinghand.com/e/7th-anatolian-school-of-catalysis-asc-7/
The 35th National Chemistry Congress with special session on Catalysis (Date: 09-12/09/2024)
Web site: https://kimya2024.com/
Don't miss out! Register now for these events and be part of the catalysis community.

Down
2. transfer of an oxygen atom from a peroxy compound to an alkene. 3. free agent in chemistry 🙂 8. degree of a reaction to yield a specific product. 9. a redox catalyst in organic synthesis 10. catalyst used to reduce organic sulfur compounds.
Accross
1. what laccase is 4. type of Lewis acid catalyst used in acylations 5. able to bind to or accept an electron or other species. 6. electrocatalyst for oxidation of primary alcohols in alkaline media 7. where lighter valuable products are formed.
Last week’s puzzle
1:TURNOVERNUMBER The maximum number of substrate molecules a single active site on a catalyst can convert into products per unit time under specific conditions.
2:LANGMUIRHINSHELWOOD Catalytic cycle where intermediates accumulate on the catalyst surface
3:RHODIUM Transition metal catalysts often used in hydrogenation and oxidation reactions
4:MICHAELISMENTEN Mathematical model relating reaction rate to catalyst concentration and kinetic parameters
5:POISONING Catalyst deactivation caused by strong adsorption of reactants
6:BET is commonly used to evaluate the gas adsorption data and generate a specific surface area
7:SINTERING Catalyst deactivation due to agglomeration of metal particles
8:ALUMINA Catalyst support material with high thermal stability and surface area
9:LEWIS Site on a catalyst surface with specific electronic properties for selective reactions
10:ASYMMETRIC Type of catalyst with chiral properties leading to enantioselective reactions
11:GRUBBS Transition metal cluster catalyst used in olefin metathesis reactions
12:MESOPOROUS Type of heterogeneous catalyst with high surface area and controlled pore size
13:XPS Surface science technique used to analyze the composition and structure of catalyst surfaces
14:PEPSIN Enzyme found in the stomach that helps digest protein
15:HOMOGENEOUS Type of catalysis where the catalyst and reactants are in the same phase

